Suppression of corrosion on stainless steel 303 with automatic impressed current cathodic protection (a-ICCP) method in simulated seawater
DOI:
https://doi.org/10.15587/1729-4061.2022.267264Keywords:
corrosion rate, impressed current cathodic protection (ICCP), simulated seawater, stainless steel 303Abstract
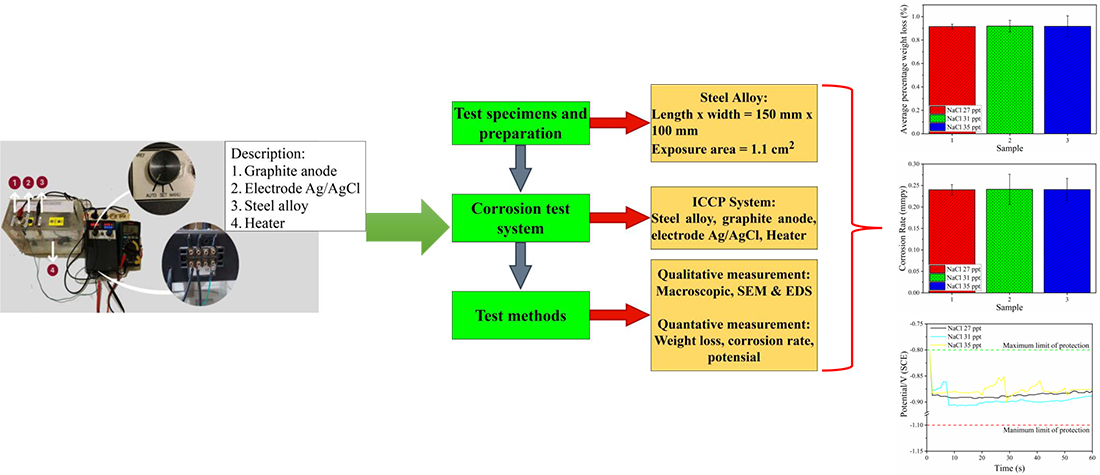
One effective method to slow down metal corrosion rate is the impressed current cathodic protection (ICCP) system. The ICCP system is suitable for coastal applications such as piping systems and offshore structures. In this application, metal surfaces tend to be exposed to seawater. Specific concentrations of seawater can accelerate the occurrence of corrosion of metals, even though they are stainless steel types. This study applied the automatic ICCP system to stainless steel 303. Stainless steel 303 will be immersed in simulated seawater at several concentrations of NaCl (27 ppt, 31 ppt, and 35 ppt). The specimens were immersed in NaCl solution for three weeks or about 504 hours at a constant temperature of 38 °C. After the sample has been soaked, quantitative and qualitative measurements were carried out. Quantitative measures include average weight loss, corrosion rate, and potential value. At the same time, the qualitative measurements include macroscopic, Scanning Electron Microscopy (SEM), and Energy Dispersive X-Ray Spectroscopy (EDS). Based on quantitative measures, it was found that the difference in average weight loss and corrosion rate for each NaCl concentration was not very significant. The difference of each parameter is less than 0.1 % and 0.22 %, respectively. The potential value quickly reaches a steady state at NaCl concentrations of 27 ppt and 31 ppt in less than 10 seconds. The results of the SEM test showed a change in the metal structure. The oxygen (O) content in the metal after the EDS test showed a decrease in this element up to 35 % at a NaCl concentration of 35 ppt. The decrease in oxygen (O) can slow down the corrosion rate in metals when exposed to seawater.
Supporting Agency
- This research is supported by the Mechanical Engineering Laboratory, Hasanuddin University, the Jakarta National Nuclear Energy Agency laboratory, and the Mechanical Engineering Laboratory, Sepuluh November Institute Surabaya.
References
- Roberge, P. R. (2012). Handbook of corrosion engineering. McGraw-Hill Education. Available at: https://www.accessengineeringlibrary.com/content/book/9780071750370
- Xiao, J., Chaudhuri, S. (2011). Predictive modeling of localized corrosion: An application to aluminum alloys. Electrochimica Acta, 56 (16), 5630–5641. doi: https://doi.org/10.1016/j.electacta.2011.04.019
- Roberge, P. R. (2008). Corrosion engineering. McGraw-Hill. Available at: https://www.accessengineeringlibrary.com/content/book/9780071482431
- Technical Handbook of Stainless Steels (2021). Atlas Steels. Available at: https://atlassteels.com.au/wp-content/uploads/2021/08/Atlas-Steels-Technical-Handbook-of-Stainless-Steels-12-08-21.pdf
- Troconis, B. C., Sharp, S. R., Ozyildirim, H. C., Demarest, C. R., Wright, J., Scully, J. R. (2020). Corrosion-resistant stainless steel strands for prestressed bridge piles in marine atmospheric environments. Available at: https://trid.trb.org/view/1693224
- Kaban, A. P. S., Ridhova, A., Priyotomo, G., Elya, B., Maksum, A., Sadeli, Y. et al. (2021). Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. Eastern-European Journal of Enterprise Technologies, 2 (6 (110)), 6–20. doi: https://doi.org/10.15587/1729-4061.2021.224435
- Bai, G., Lu, S., Li, D., Li, Y. (2016). Influences of niobium and solution treatment temperature on pitting corrosion behaviour of stabilised austenitic stainless steels. Corrosion Science, 108, 111–124. doi: https://doi.org/10.1016/j.corsci.2016.03.009
- Loto, R. T. (2013). Pitting corrosion evaluation of austenitic stainless steel type 304 in acid chloride media. Journal of Materials and Environmental Science, 4 (4), 448–459. Available at: https://www.researchgate.net/publication/272621606_Pitting_corrosion_evaluation_of_austenitic_stainless_steel_type_304_in_acid_chloride_media
- Loto, R. T., Loto, C. A., Popoola, A. P. I., Ranyaoa, M. (2012). Corrosion resistance of austenitic stainless steel in sulphuric acid. International Journal of Physical Sciences, 7 (10). doi: https://doi.org/10.5897/ijps11.1580
- Iliyasu, I., Yawas, D. S., Aku, S. Y. (2012). Corrosion behavior of austenitic stainless steel in sulphuric acid at various concentrations. Advances in Applied Science Research, 3 (6), 3909–3915. Available at: https://www.primescholars.com/articles/corrosion-behavior-of-austenitic-stainless-steel-in-sulphuric-acid-atvarious-concentrations.pdf
- Xu, L., Xin, Y., Ma, L., Zhang, H., Lin, Z., Li, X. (2021). Challenges and solutions of cathodic protection for marine ships. Corrosion Communications, 2, 33–40. doi: https://doi.org/10.1016/j.corcom.2021.08.003
- Bahekar, P. V., Gadve, S. S. (2017). Impressed current cathodic protection of rebar in concrete using Carbon FRP laminate. Construction and Building Materials, 156, 242–251. doi: https://doi.org/10.1016/j.conbuildmat.2017.08.145
- Evgeny, B., Hughes, T., Eskin, D. (2016). Effect of surface roughness on corrosion behaviour of low carbon steel in inhibited 4 M hydrochloric acid under laminar and turbulent flow conditions. Corrosion Science, 103, 196–205. doi: https://doi.org/10.1016/j.corsci.2015.11.019
- Zheng, Z. B., Zheng, Y. G., Zhou, X., He, S. Y., Sun, W. H., Wang, J. Q. (2014). Determination of the critical flow velocities for erosion–corrosion of passive materials under impingement by NaCl solution containing sand. Corrosion Science, 88, 187–196. doi: https://doi.org/10.1016/j.corsci.2014.07.043
- Liang, J., Deng, A., Xie, R., Gomez, M., Hu, J., Zhang, J. et al. (2013). Impact of flow rate on corrosion of cast iron and quality of re-mineralized seawater reverse osmosis (SWRO) membrane product water. Desalination, 322, 76–83. doi: https://doi.org/10.1016/j.desal.2013.05.001
- Vasyliev, G. S. (2015). The influence of flow rate on corrosion of mild steel in hot tap water. Corrosion Science, 98, 33–39. doi: https://doi.org/10.1016/j.corsci.2015.05.007
- Kim, Y.-S., Seok, S., Lee, J.-S., Lee, S. K., Kim, J.-G. (2018). Optimizing anode location in impressed current cathodic protection system to minimize underwater electric field using multiple linear regression analysis and artificial neural network methods. Engineering Analysis with Boundary Elements, 96, 84–93. doi: https://doi.org/10.1016/j.enganabound.2018.08.012
- Lauria, D., Minucci, S., Mottola, F., Pagano, M., Petrarca, C. (2018). Active cathodic protection for HV power cables in undersea application. Electric Power Systems Research, 163, 590–598. doi: https://doi.org/10.1016/j.epsr.2017.11.016
- Jeong, J. A., Jin, C. K. (2014). Experimental Studies of Effectiveness of Hybrid Cathodic Protection System on the Steel in Concrete. Science of Advanced Materials, 6 (10), 2165–2170. doi: https://doi.org/10.1166/sam.2014.2061
- Wilson, K., Jawed, M., Ngala, V. (2013). The selection and use of cathodic protection systems for the repair of reinforced concrete structures. Construction and Building Materials, 39, 19–25. doi: https://doi.org/10.1016/j.conbuildmat.2012.05.037
- Qiao, G., Guo, B., Ou, J. (2017). Numerical Simulation to Optimize Impressed Current Cathodic Protection Systems for RC Structures. Journal of Materials in Civil Engineering, 29 (6). doi: https://doi.org/10.1061/(asce)mt.1943-5533.0001837
- Zhu, J.-H., Wei, L., Moahmoud, H., Redaelli, E., Xing, F., Bertolini, L. (2017). Investigation on CFRP as dual-functional material in chloride-contaminated solutions. Construction and Building Materials, 151, 127–137. doi: https://doi.org/10.1016/j.conbuildmat.2017.05.213
- Christodoulou, C., Glass, G., Webb, J., Austin, S., Goodier, C. (2010). Assessing the long term benefits of Impressed Current Cathodic Protection. Corrosion Science, 52 (8), 2671–2679. doi: https://doi.org/10.1016/j.corsci.2010.04.018
- Li, S., Zhang, L., Wang, Y., Hu, P., Jiang, N., Guo, P. et al. (2021). Effect of cathodic protection current density on corrosion rate of high-strength steel wires for stay cable in simulated dynamic marine atmospheric rainwater. Structures, 29, 1655–1670. doi: https://doi.org/10.1016/j.istruc.2020.12.028
- Jusoh, S. M., Nik, W. M. N. W., Azman, N. A., Zulkifli, M. F. R. (2020). Corrosion Behavior of Low-Carbon Steel and Stainless Steel 304 Under Two Soil Conditions at Pantai Mengabang Telipot, Terengganu, Malaysia. Malaysian Journal of Analytical Sciences, 24 (6), 954–969. Available at: https://mjas.analis.com.my/mjas/v24_n6/pdf/Suriani_24_6_14.pdf
- Thomas, S., Ott, N., Schaller, R. F., Yuwono, J. A., Volovitch, P., Sundararajan, G. et al. (2016). The effect of absorbed hydrogen on the dissolution of steel. Heliyon, 2 (12), e00209. doi: https://doi.org/10.1016/j.heliyon.2016.e00209
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Hamsir, Onny Sutresman, Hairul Arsyad, Muhammad Syahid, Agus Widyianto

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.









