Development of a resource-saving technology for the treatment of ferrum-containing wastewater from etching operations
DOI:
https://doi.org/10.15587/1729-4061.2022.267949Keywords:
resource-saving technology, etching solutions, rum-containing impurities, magnetic device, reagent consumptionAbstract
The object of this study is wastewater from chloride and sulfate etching operations.
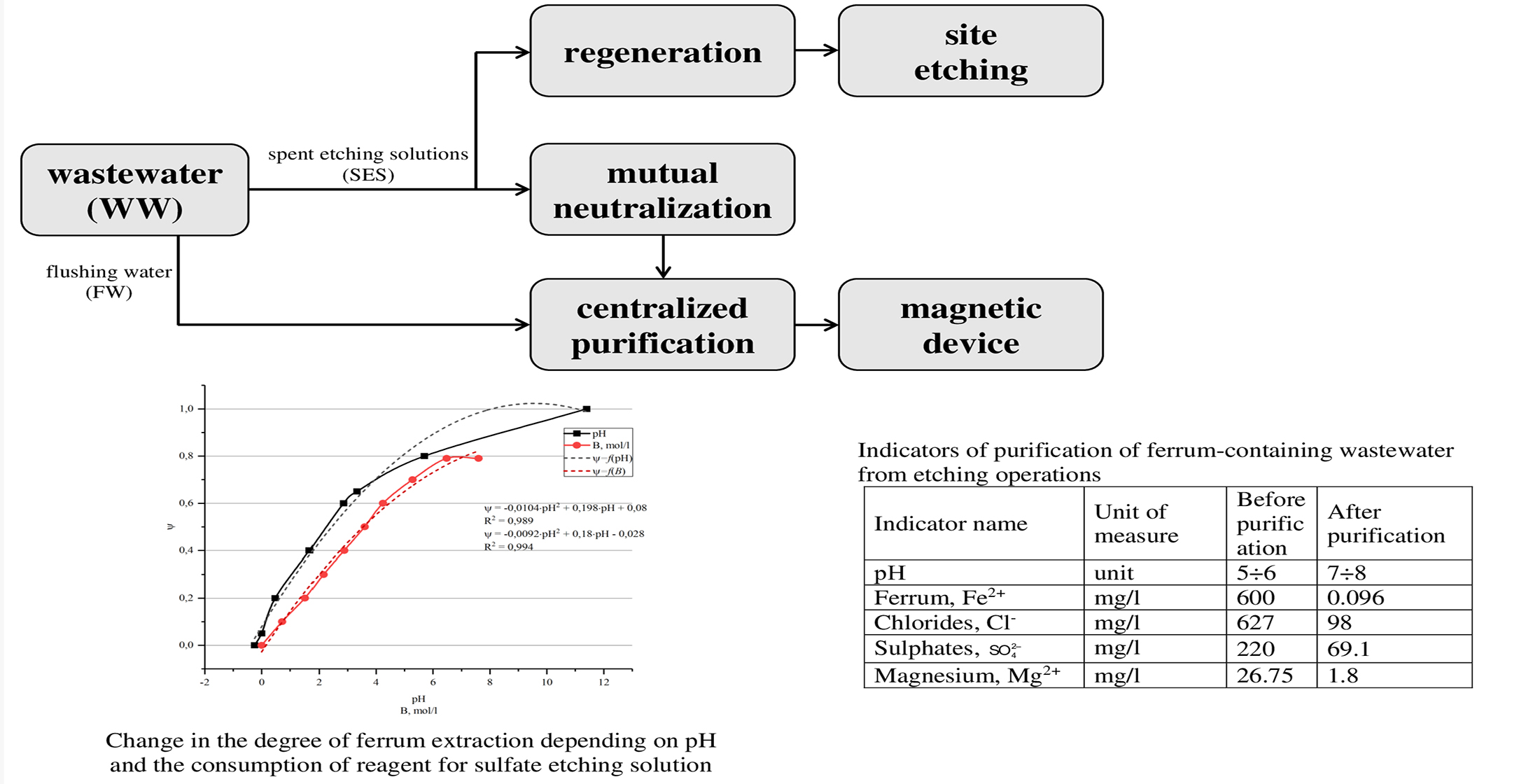
This paper reports results of research on ways to reduce the consumption of chemical reagents in wastewater treatment systems from etching operations. Spent etching solutions are subject to regeneration with return to the production process and partial dosing to the main stream of wastewater. It was found that at a ferrum concentration of 30 g/l in etching solutions, the solution must be treated with an alkaline reagent (10–20 % NaOH) to pH=3.5–4.0 in order to return to the technological process. In this case, the final concentration of ferrum is 11 g/l. The use of hydrogen peroxide (20–40 % H2O2) together with the alkaline reagent makes it possible to increase the degree of extraction by 30 %, that is, the final concentration of ferrum is 8 g/l. When discharging 1 m3 of etching solutions, 0.5 m3 is subject to regeneration and, after mixing with 0.5 m3 of the commercial reagent (HCl) it returns to the technological process. Commercial acid consumption is reduced by 50 %. It was shown that the use of individual flows of waste solutions as a chemical reagent reduces the cost of reagents for their neutralization (saving alkaline reagent is 80 %). Thus, 1.2 kg/m3 of a commercially available reagent (NaOH) is consumed per 1 m3 of solutions (etching and degreasing) after mixing them, and, without mutual neutralization, this consumption is 6 kg/m3. To neutralize etching solutions, it is recommended to carry out the process in the range of pH=6.5–7.5. For a solution in which Fe3+ ions predominate with an initial concentration of 0.53 mol/L, a degree of extraction of 0.9 is achieved, and the total consumption of the reagent (7.1 mol/l) exceeds the stoichiometric one by only 10 %.
Deep purification from ferrum-containing impurities using a magnetic device expands the possibilities of practical implementation of further desalting with inverse osmosis.
References
- Korchik, N. M., Belikova, S. V. (2012). Ochistka i regeneratsiya stochnykh vod gal'vanicheskogo proizvodstva. Ekologiya plyus. Nauchno-proizvodstvenniy ekologicheskiy zhurnal, 6 (33), 10–13.
- Cheremisin, A. V., Valiullin, L. R., Myazin, N. S., Logunov, S. E. (2021). Efficient treatment of wastewater from galvanic plants. Journal of Physics: Conference Series, 1942 (1), 012095. doi: https://doi.org/10.1088/1742-6596/1942/1/012095
- Liu, Q., Pan, D., Ding, T., Ye, M., He, F. (2020). Clean & environmentally friendly regeneration of Fe-surface cleaning pickling solutions. Green Chemistry, 22 (24), 8728–8733. doi: https://doi.org/10.1039/d0gc03297b
- Yatskov, M., Korchyk, N., Budenkova, N., Kyrylyuk, S., Prorok, O. (2017). Development of technology for recycling the liquid iron-containing wastes of steel surface etching. Eastern-European Journal of Enterprise Technologies, 2 (6 (86)), 70–77. doi: https://doi.org/10.15587/1729-4061.2017.97256
- Garashchenko, V. I., Garashchenko, A. V., Luk’yanchuk, A. P. (2012). The precipitation of the dispersed phase of liquid medium impurities in a magnetized ferrito-ferromagnetic nozzle. Russian Journal of Physical Chemistry A, 86 (4), 685–688. doi: https://doi.org/10.1134/s0036024412040085
- Yatskov, M. V., Mysina, O. I. (2001). Pat. No. 36351 UA. Device for removal of magnetic and non-magnetic inclusions from liquid. No. 99126648; declareted: 07.12.1999; published: 16.04.2001, Bul. No. 3. Available at: https://uapatents.com/3-36351-pristrijj-dlya-ochishhennya-ridini-vid-magnitnikh-ta-nemagnitnikh-vklyuchen.html
- Merentsov, N. A., Bokhan, S. A., Lebedev, V. N., Persidskiy, A. V., Balashov, V. A. (2018). System for Centralised Collection, Recycling and Removal of Waste Pickling and Galvanic Solutions and Sludge. Materials Science Forum, 927, 183–189. doi: https://doi.org/10.4028/www.scientific.net/msf.927.183
- Kochetov, G. M. (2000). Kompleksnaya ochistka stochnykh vod promyshlennykh predpriyatiy s regeneratsiey tyazhelykh metallov. Ekotekhnologii i resursosberezhenie, 4, 41–43.
- Cunha, T. N. D., Trindade, D. G., Canesin, M. M., Effting, L., de Moura, A. A., Moisés, M. P. et al. (2020). Reuse of Waste Pickling Acid for the Production of Hydrochloric Acid Solution, Iron(II) Chloride and Magnetic Iron Oxide: An Eco-Friendly Process. Waste and Biomass Valorization, 12 (3), 1517–1528. doi: https://doi.org/10.1007/s12649-020-01079-1
- Uretskiy, E. A. (2007). Resursosberegayuschie tekhnologii v vodnom khozyaystve promyshlennykh predpriyatiy. Brest: BrGTU, 396.
- Xiaoyu, W., Gang, L., Shuo, Y. (2020). Study on the Treatment and Recovery of Acid in Steel Pickling Wastewater with Diffusion Dialysis. IOP Conference Series: Earth and Environmental Science, 510 (4), 042046. doi: https://doi.org/10.1088/1755-1315/510/4/042046
- Serdiuk, V. O. (2021). Membranni elektrokhimichni prystroi v protsesakh reheneratsiyi halvanichnykh rozchyniv. Sumy: SDU, 192. Available at: https://essuir.sumdu.edu.ua/handle/123456789/86159
- Tevtul, Ya. Yu., Nechyporenko, O. V., Makh, N. Ya., Mykhaletska, O. M. (2008). Elektrokhimichna membranna reheneratsiya khlorydnykh rozchyniv travlennia midi. Ukraynskyi khymycheskyi zhurnal, 74 (2), 97–101. Available at: http://dspace.nbuv.gov.ua/handle/123456789/14594
- Koltyshev, S. M. et al. (2006). Opyt ochistki ot parov solyanoy kisloty aspiratsionnogo vozdukha travil'nogo otdeleniya. Stal', 2, 77–78.
- Vasylenko, I. A., Kumaniov, S. O. (2011). Pat. No.100944 UA. Process for the preparation of modified yellow iron oxide. No. 201112246; declareted: 19.10.2011; published: 11.02.2013, Bul. No. 3. Available at: https://base.uipv.org/searchINV/search.php?action=viewdetails&IdClaim=182968
- Pietrelli, L., Ferro, S., Vocciante, M. (2018). Raw materials recovery from spent hydrochloric acid-based galvanizing wastewater. Chemical Engineering Journal, 341, 539–546. doi: https://doi.org/10.1016/j.cej.2018.02.041
- Sharma, V. K., Yngard, R. A., Cabelli, D. E., Clayton Baum, J. (2008). Ferrate(VI) and ferrate(V) oxidation of cyanide, thiocyanate, and copper(I) cyanide. Radiation Physics and Chemistry, 77 (6), 761–767. doi: https://doi.org/10.1016/j.radphyschem.2007.11.004
- Baran, B. A., Bubenshchykova, H. T., Khriashchevskyi, V. M. (2010). Antropohenne zabrudnennia vody ta sposoby yii ochyshchennia. Visnyk Khmelnytskoho natsionalnoho universytetu, 2, 234–237. Available at: http://journals.khnu.km.ua/vestnik/pdf/tech/2010_2/44bar.pdf
- Korzhik, N. N., Garniy, A. I., Shevchenko, V. E., Khaskin, V. Yu., Kostash, S. M. (2017). Primenenie mikrodugovoy obrabotki vo vraschayuschikhsya magnitnykh polyakh dlya ochistki zagryaznennykh i stochnykh vod. Mezhdunarodnyy Nauchnyy Institut “Educatio” : Tekhnicheskie nauki, I (26), 17–27.
- Kochetov, G., Samchenko, D., Lastivka, O., Derecha, D. (2022). Determining the rational parameters for processing spent etching solutions by ferritization using alternating magnetic fields. Eastern-European Journal of Enterprise Technologies, 3 (10 (117)), 21–28. doi: https://doi.org/10.15587/1729-4061.2022.259791
- Garashchenko, I. V., Garashchenko, V. I., Astrelin, I. M. (2019). Magnetosorption purification of liquid chemical products from ferromagnetic impurities. Voprosy Khimii i Khimicheskoi Tekhnologii, 1, 80–85. doi: https://doi.org/10.32434/0321-4095-2019-122-1-80-85
- Mehta, D., Mazumdar, S., Singh, S. K. (2015). Magnetic adsorbents for the treatment of water/wastewater – A review. Journal of Water Process Engineering, 7, 244–265. doi: https://doi.org/10.1016/j.jwpe.2015.07.001
- Korchyk, N. M., Yatskov, M. V., Bielikova, S. V. (2012). Pat. No, 76053 UA. Process for the purification of waste water of electroplating industry. No. u201206086; declareted: 21.05.2012; published: 25.12.2012, Bul. No, 24. Available at: https://uapatents.com/6-76053-sposib-ochishhennya-stichnikh-vod-galvanichnogo-virobnictva.html
- Kyryliuk, S. V. (2017). Ochyshchennia kontsentrovanykh stichnykh vod halvanichnoho vyrobnytstva u kombinovanii systemi. Rivne: NUVHP, 206.
- Yatskov, M., Korchyk, N., Mysina, O., Budenkova, N. (2021). Improvement of the technological treatment scheme of iron-containing wastewater from etching operations. EUREKA: Life Sciences, 3, 21–28. doi: https://doi.org/10.21303/2504-5695.2021.001883
- Yatskov, M., Korchyk, N., Mysina, O., Budenkova, N. (2021). Creation of a combined system for treatment of iron-containing wastewater from etching operations. Technology Audit and Production Reserves, 6 (3 (62)), 21–26. doi: https://doi.org/10.15587/2706-5448.2021.247550
- Yatskov, M. V., Korchyk, N. M., Prorok, O. A., Besediuk, V. Yu. (2020). Pat. No. 147127. Sposib vyluchennia khromu iz vysokokontsentrovanykh vidkhodiv shkirzavodiv. No. 202006909; declareted: 28.10.2020; published: 14.04.2021, Bul. No. 15. Available at: https://base.uipv.org/searchINV/search.php?action=viewdetails&IdClaim=275516
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Mykola Yatskov, Natalia Korchyk, Nadia Budenkova, Oksana Mysina

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.