Improvement of fatty systems biotechnological interesterification with immobilized enzyme preparation usage
DOI:
https://doi.org/10.15587/1729-4061.2022.268373Keywords:
biotechnological interesterification, immobilized enzyme preparation, interesterification duration, interesterified fat quality indicatorsAbstract
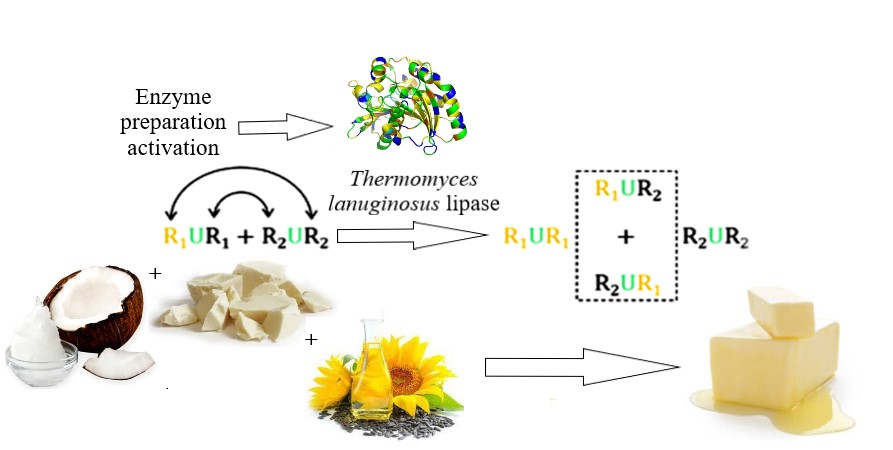
This work research object was fat systems interesterification biotechnology using the Lipozyme TL IM immobilized enzyme preparation. The problem of enzyme preparation activation by moistening with sodium bicarbonate aqueous solution with 7.4 ... 7.7 (3 % wt.) pH was solved in the work. The obtained results made it possible to minimize the interesterification process duration with high-quality product obtaining. The proposed enzyme preparation processing made it possible to reduce the duration of the biointeresterification process in a model fat mixture (palm stearin, coconut and soybean oils in a ratio of 1:1:1, respectively) to 3.5...3.7 hours. The product with high quality indicators, namely up to 0.26 mg KOH/g acid number, up to 0.60 mmol ½ O/kg peroxide number and 1.70 c.u. anisidine number, was obtained as a result. The obtained data can be explained by a fact that effective biocatalysis with lipolytic enzymes as the protein molecules requires the existence of two phases – lipid and water. This fact was provided by the activation parameters justified in the study. The obtained results feature was possibility of enzyme preparation activation, which is not provided under industrial conditions due to the threat of raw materials and finished products hydrolytic processes, which leads to the finished product quality deterioration. The research results made it possible to minimize hydrolytic processes in fat system during interesterification with simultaneous process efficiency increase. From a practical point of view, the discovered activation mechanism made it possible to adjust the enzyme preparation processing conditions in fat systems interesterification technology. The applied aspect of scientific result using was the possibility of improving the typical technological process of fat interesterification
References
- Belinska, A., Bochkarev, S., Varankina, O., Rudniev, V., Zviahintseva, O., Rudnieva, K. et al. (2019). Research on oxidative stability of protein-fat mixture based on sesame and flax seeds for use in halva technology. Eastern-European Journal of Enterprise Technologies, 5 (11 (101)), 6–14. doi: https://doi.org/10.15587/1729-4061.2019.178908
- Kumar, A., Dhar, K., Kanwar, S. S., Arora, P. K. (2016). Lipase catalysis in organic solvents: advantages and applications. Biological Procedures Online, 18 (1). doi: https://doi.org/10.1186/s12575-016-0033-2
- Sytnik, N., Demidov, I., Kunitsa, E., Mazaeva, V., Chumak, O. (2016). A study of fat interesterification parameters’ effect on the catalytic reaction activity of potassium glycerate. Eastern-European Journal of Enterprise Technologies, 3 (6 (81)), 33–38. doi: https://doi.org/10.15587/1729-4061.2016.71236
- Remonatto, D., Miotti Jr., R. H., Monti, R., Bassan, J. C., de Paula, A. V. (2022). Applications of immobilized lipases in enzymatic reactors: A review. Process Biochemistry, 114, 1–20. doi: https://doi.org/10.1016/j.procbio.2022.01.004
- Xie, W., Zang, X. (2016). Immobilized lipase on core–shell structured Fe3O4–MCM-41 nanocomposites as a magnetically recyclable biocatalyst for interesterification of soybean oil and lard. Food Chemistry, 194, 1283–1292. doi: https://doi.org/10.1016/j.foodchem.2015.09.009
- Meunier, S. M., Kariminia, H.-R., Legge, R. L. (2017). Immobilized Enzyme Technology for Biodiesel Production. Advances in Biofeedstocks and Biofuels, 67–106. doi: https://doi.org/10.1002/9781119117551.ch3
- Zhang, H., Secundo, F., Sun, J., Mao, X. (2022). Advances in enzyme biocatalysis for the preparation of functional lipids. Biotechnology Advances, 61, 108036. doi: https://doi.org/10.1016/j.biotechadv.2022.108036
- Kutluk, T., Gürkaya Kutluk, B. (2022). A commercial lipase Resinase® HT (Aspergillus oryzae) efficiency on triglycerides transesterification and process optimization. Sustainable Chemistry and Pharmacy, 30, 100862. doi: https://doi.org/10.1016/j.scp.2022.100862
- Fernández, A., Longo, M. A., Deive, F. J., Álvarez, M. S., Rodríguez, A. (2022). Dual role of a natural deep eutectic solvent as lipase extractant and transesterification enhancer. Journal of Cleaner Production, 346, 131095. doi: https://doi.org/10.1016/j.jclepro.2022.131095
- Sharma, S., Bhatt, R. (2021). Enhanced production of Commercially Important Amylolytic Enzyme. Lambert Academic Publishing.
- Samoylova, Y. V., Piligaev, A. V., Sorokina, K. N., Rozanov, A. S., Peltek, S. E., Novikov, A. A. et al. (2016). Application of the immobilized bacterial recombinant lipase from Geobacillus stearothermophilus G3 for the production of fatty acid methyl esters. Catalysis in Industry, 8 (2), 187–193. doi: https://doi.org/10.1134/s2070050416020082
- Pinheiro, B. B., Rios, N. S., Rodríguez Aguado, E., Fernandez-Lafuente, R., Freire, T. M., Fechine, P. B. A. et al. (2019). Chitosan activated with divinyl sulfone: a new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. International Journal of Biological Macromolecules, 130, 798–809. doi: https://doi.org/10.1016/j.ijbiomac.2019.02.145
- Ismail, A. R., Kashtoh, H., Baek, K.-H. (2021). Temperature-resistant and solvent-tolerant lipases as industrial biocatalysts: Biotechnological approaches and applications. International Journal of Biological Macromolecules, 187, 127–142. doi: https://doi.org/10.1016/j.ijbiomac.2021.07.101
- Patzl-Fischerleitner, E., Eder, R. (2009). Determination of enzymatic activities of commercial enzyme preparations. Mitteilungen Klosterneuburg, 59 (1), 8–14. Available at: https://www.weinobst.at/dam/jcr:89c04c6b-dc0d-427b-87bb-c23fd5708a14/8-2009.pdf
- Peng, B., Chen, F., Liu, X., Hu, J.-N., Zheng, L.-F., Li, J., Deng, Z.-Y. (2020). Trace water activity could improve the formation of 1,3-oleic-2-medium chain-rich triacylglycerols by promoting acyl migration in the lipase RM IM catalyzed interesterification. Food Chemistry, 313, 126130. doi: https://doi.org/10.1016/j.foodchem.2019.126130
- Osorio, N. M., da Fonseca, M. R., Ferreira-Dias, S. (2006). Operational stability of Thermomyces lanuginosa lipase during interesterification of fat in continuous packed-bed reactor. European Journal of Lipid Science and Technology, 108 (7), 545–553. doi: https://doi.org/10.1002/ejlt.200600029
- Zhang, Z., Lee, W. J., Sun, X., Wang, Y. (2022). Enzymatic interesterification of palm olein in a continuous packed bed reactor: Effect of process parameters on the properties of fats and immobilized Thermomyces lanuginosus lipase. LWT, 162, 113459. doi: https://doi.org/10.1016/j.lwt.2022.113459
- Nekrasov, P. O., Gudz, O. M., Nekrasov, O. P., Berezka, T. O. (2020). Optimizing the parameters of the production process of fat systems with a minimum content of trans-isomers. Voprosy khimii i khimicheskoi tekhnologii, 3, 128–133. doi: https://doi.org/10.32434/0321-4095-2020-130-3-128-133
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Anna Belinska, Olga Bliznjuk, Olena Shcherbak, Nataliia Masalitina, Liliia Myronenko, Oleksandra Varankina, Serhii Samoilenko, Viktoriia Borovkova, Natalya Kibenko, Valentina Timchenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.









