Synthesis of nanocomposites reduced graphene oxide-silver nanoparticles prepared by hydrothermal technique using sodium borohydride as a reductor for photocatalytic degradation of Pb ions in aqueous solution
DOI:
https://doi.org/10.15587/1729-4061.2022.269844Keywords:
lead, reduced graphene oxide, silver nanoparticles, rGO/AgNPs nanocomposite, sodium borohydrateAbstract
Heavy metals are pollutants that are harmful to living things and the environment can be degraded by microbes or understood by other living things so that they can cause health problems. One of the heavy metals that is often found in wastewater is lead. Lead is widely used in the manufacture of batteries, metal products such as ammunition, cable coatings, Polyvinyl Chloride (PVC) tubing, solder, chemicals and dyes
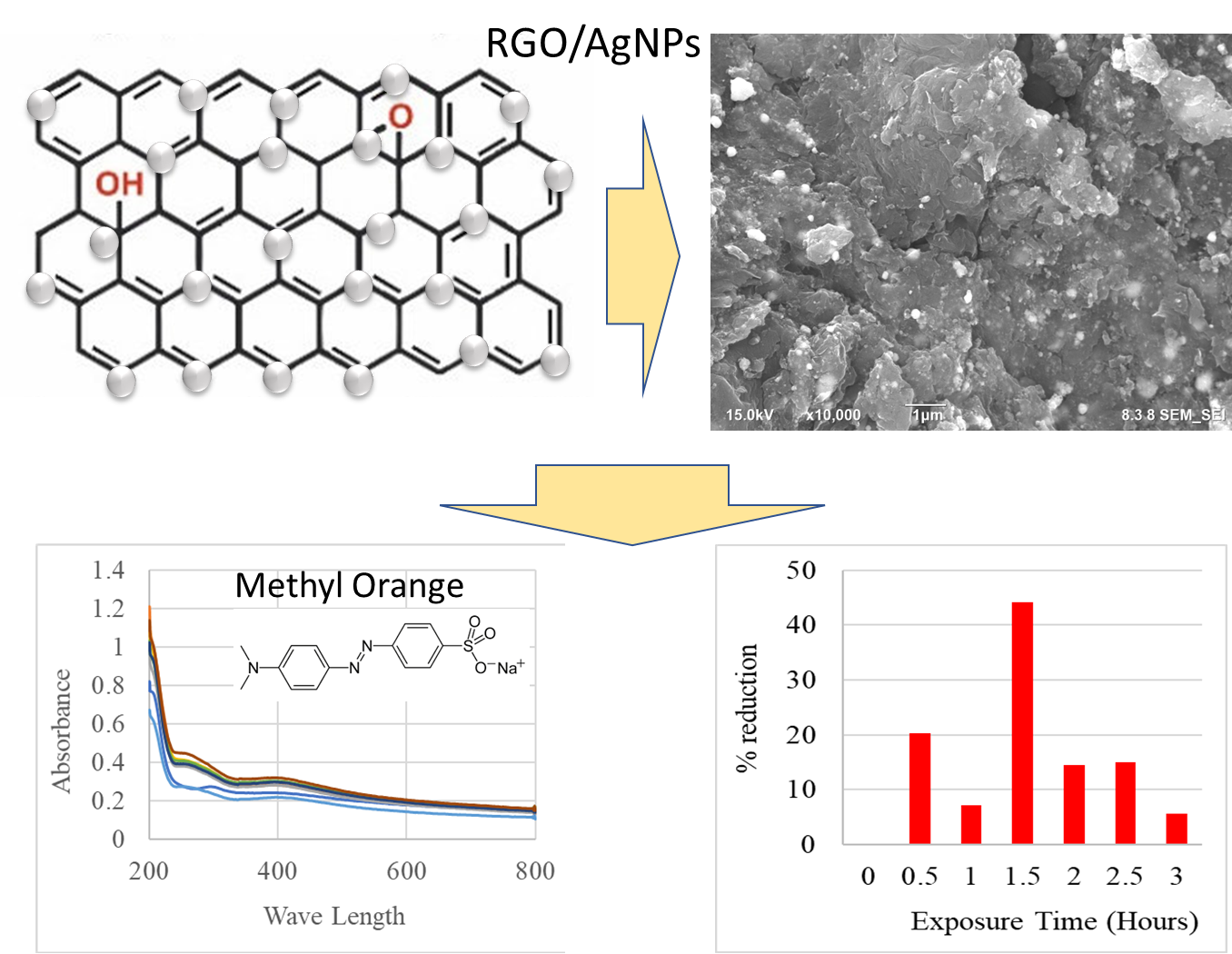
This use causes humans to be exposed to large amounts of lead. One method to deal with lead pollution is to use photocatalysts. Photocatalysts react with heavy metals and reduce them so that the level of toxicity becomes lower than before through photocatalytic reactions. In this study, synthesis of reduced graphene oxide/silver nanoparticle nanoparticles was performed by facile hydrothermal methods for photocatalytic degradation of Pb ion. The characterization results indicate that the synthesis has been successfully carried out. The successful result of rGO/AgNPs nanocomposites synthesis was proved by several techniques such as X-ray diffraction analysis, Raman, UV-Vis spectroscopy, Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray analysis (EDX). This indicates the presence of these groups in the graphene oxide and rGO/AgNPs samples, respectively. The resulting rGO/AgNPs nanocomposite has an absorbance peak at a wavelength of 267 nm. The diffraction peaks for nanocomposites rGO/AgNPs and their Miller indices were 38.08° (111), 44.16° (200), 64.44° (220), and 77.44° (311). The Raman spectra of rGO/AgNPs exhibits D bands at 1334,13 with intensity of 630,60 cm−1 and G band at 1594,61 with intensity of 477,29 cm−1. The ID/IG ratio rGO/AgNPs-NaBH4 is ~1,32. Furthermore, the photocatalytic activity test results showed that the rGO/AgNPs nanocomposite was able to reduce Pb2+ to Pb with a maximum exposure time of 1.5 hours
Supporting Agency
- The research was carried out using laboratories at the Metallurgical Research Center, National Innovation Research Agency (BRIN) and material characterization from the analytical instrumentation facility ELSA (E-Layanan Sains).
References
- Masindi, V., Muedi, K. L. (2018). Environmental Contamination by Heavy Metals. Heavy Metals. doi: https://doi.org/10.5772/intechopen.76082
- Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7 (2), 60–72. doi: https://doi.org/10.2478/intox-2014-0009
- Litter, M. I. (2015). Mechanisms of removal of heavy metals and arsenic from water by TiO2-heterogeneous photocatalysis. Pure and Applied Chemistry, 87 (6), 557–567. doi: https://doi.org/10.1515/pac-2014-0710
- Gusain, R., Kumar, N., Ray, S. S. (2020). Factors Influencing the Photocatalytic Activity of Photocatalysts in Wastewater Treatment. Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment, 229–270. doi: https://doi.org/10.1002/9781119631422.ch8
- Fröschl, T., Hörmann, U., Kubiak, P., Kučerová, G., Pfanzelt, M., Weiss, C. K. et al. (2012). High surface area crystalline titanium dioxide: potential and limits in electrochemical energy storage and catalysis. Chemical Society Reviews, 41 (15), 5313. doi: https://doi.org/10.1039/c2cs35013k
- Setvín, M., Aschauer, U., Scheiber, P., Li, Y.-F., Hou, W., Schmid, M. et al. (2013). Reaction of O2 with subsurface oxygen vacancies on TiO2 anatase (101). Science, 341 (6149), 988–991. doi: https://doi.org/10.1126/science.1239879
- Wang, L., Wei, H., Fan, Y., Liu, X., Zhan, J. (2009). Synthesis, Optical Properties, and Photocatalytic Activity of One-Dimensional CdS@ZnS Core-Shell Nanocomposites. Nanoscale Research Letters, 4 (6). doi: https://doi.org/10.1007/s11671-009-9280-3
- Chen, P., Wang, F., Chen, Z.-F., Zhang, Q., Su, Y., Shen, L. et al. (2017). Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: The significant roles of reactive oxygen species. Applied Catalysis B: Environmental, 204, 250–259. doi: https://doi.org/10.1016/j.apcatb.2016.11.040
- Lv, N., Li, Y., Huang, Z., Li, T., Ye, S., Dionysiou, D. D., Song, X. (2019). Synthesis of GO/TiO2/Bi2WO6 nanocomposites with enhanced visible light photocatalytic degradation of ethylene. Applied Catalysis B: Environmental, 246, 303–311. doi: https://doi.org/10.1016/j.apcatb.2019.01.068
- Fan, W., Zhou, Z., Wang, W., Huo, M., Zhang, L., Zhu, S. et al. (2019). Environmentally friendly approach for advanced treatment of municipal secondary effluent by integration of micro-nano bubbles and photocatalysis. Journal of Cleaner Production, 237, 117828. doi: https://doi.org/10.1016/j.jclepro.2019.117828
- Kumar, A., Khan, M., Fang, L., Lo, I. M. C. (2019). Visible-light-driven N-TiO2@SiO2@Fe3O4 magnetic nanophotocatalysts: Synthesis, characterization, and photocatalytic degradation of PPCPs. Journal of Hazardous Materials, 370, 108–116. doi: https://doi.org/10.1016/j.jhazmat.2017.07.048
- Kar, P., Zeng, S., Zhang, Y., Vahidzadeh, E., Manuel, A., Kisslinger, R. et al. (2019). High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Applied Catalysis B: Environmental, 243, 522–536. doi: https://doi.org/10.1016/j.apcatb.2018.08.002
- Méndez-Medrano, M. G., Kowalska, E., Lehoux, A., Herissan, A., Ohtani, B., Bahena, D. et al. (2016). Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. The Journal of Physical Chemistry C, 120 (9), 5143–5154. doi: https://doi.org/10.1021/acs.jpcc.5b10703
- Kamat, P. V. (2011). Graphene-Based Nanoassemblies for Energy Conversion. The Journal of Physical Chemistry Letters, 2 (3), 242–251. doi: https://doi.org/10.1021/jz101639v
- Yuan, L., Zhang, C., Zhang, X., Lou, M., Ye, F., Jacobson, C. R. et al. (2019). Photocatalytic Hydrogenation of Graphene Using Pd Nanocones. Nano Letters, 19 (7), 4413–4419. doi: https://doi.org/10.1021/acs.nanolett.9b01121
- Guo, H., Jiang, N., Wang, H., Shang, K., Lu, N., Li, J., Wu, Y. (2019). Enhanced catalytic performance of graphene-TiO2 nanocomposites for synergetic degradation of fluoroquinolone antibiotic in pulsed discharge plasma system. Applied Catalysis B: Environmental, 248, 552–566. doi: https://doi.org/10.1016/j.apcatb.2019.01.052
- Russo, P., Hu, A., Compagnini, G. (2013). Synthesis, Properties and Potential Applications of Porous Graphene: A Review. Nano-Micro Letters, 5 (4), 260–273. doi: https://doi.org/10.1007/bf03353757
- Pastrana-Martínez, L. M., Morales-Torres, S., Figueiredo, J. L., Faria, J. L., Silva, A. M. T. (2018). Graphene photocatalysts. Multifunctional Photocatalytic Materials for Energy, 79–101. doi: https://doi.org/10.1016/b978-0-08-101977-1.00006-5
- Lee, C., Wei, X., Kysar, J. W., Hone, J. (2008). Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science, 321 (5887), 385–388. doi: https://doi.org/10.1126/science.1157996
- Mayorov, A. S., Gorbachev, R. V., Morozov, S. V., Britnell, L., Jalil, R., Ponomarenko, L. A. et al. (2011). Micrometer-Scale Ballistic Transport in Encapsulated Graphene at Room Temperature. Nano Letters, 11 (6), 2396–2399. doi: https://doi.org/10.1021/nl200758b
- Park, S., Ruoff, R. S. (2009). Chemical methods for the production of graphenes. Nature Nanotechnology, 4 (4), 217–224. doi: https://doi.org/10.1038/nnano.2009.58
- Morales-Torres, S., Pastrana-Martínez, L. M., Figueiredo, J. L., Faria, J. L., Silva, A. M. T. (2012). Design of graphene-based TiO2 photocatalysts – a review. Environmental Science and Pollution Research, 19 (9), 3676–3687. doi: https://doi.org/10.1007/s11356-012-0939-4
- Kamat, P. V. (2009). Graphene-Based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Carbon Support. The Journal of Physical Chemistry Letters, 1 (2), 520–527. doi: https://doi.org/10.1021/jz900265j
- Handayani, M., Mulyaningsih, Y., Aulia Anggoro, M., Abbas, A., Setiawan, I., Triawan, F. et al. (2022). One-pot synthesis of reduced graphene oxide/chitosan/zinc oxide ternary nanocomposites for supercapacitor electrodes with enhanced electrochemical properties. Materials Letters, 314, 131846. doi: https://doi.org/10.1016/j.matlet.2022.131846
- Handayani, M., Suwaji, B. I., Ihsantia Ning Asih, G., Kusumaningsih, T., Kusumastuti, Y., Rochmadi, Anshori, I. (2022). In-situ synthesis of reduced graphene oxide/silver nanoparticles (rGO/AgNPs) nanocomposites for high loading capacity of acetylsalicylic acid. Nanocomposites, 8 (1), 74–80. doi: https://doi.org/10.1080/20550324.2022.2054210
- Wang, N., Zhang, F., Mei, Q., Wu, R., Wang, W. (2020). Photocatalytic TiO2/rGO/CuO Composite for Wastewater Treatment of Cr(VI) Under Visible Light. Water, Air, & Soil Pollution, 231 (5). doi: https://doi.org/10.1007/s11270-020-04609-8
- Chong, M. N., Jin, B., Chow, C. W. K., Saint, C. (2010). Recent developments in photocatalytic water treatment technology: A review. Water Research, 44 (10), 2997–3027. doi: https://doi.org/10.1016/j.watres.2010.02.039
- Sarina, S., Waclawik, E. R., Zhu, H. (2013). Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chemistry, 15 (7), 1814. doi: https://doi.org/10.1039/c3gc40450a
- Tarcan, R., Todor-Boer, O., Petrovai, I., Leordean, C., Astilean, S., Botiz, I. (2020). Reduced graphene oxide today. Journal of Materials Chemistry C, 8 (4), 1198–1224. doi: https://doi.org/10.1039/c9tc04916a
- Latiff, N. M., Fu, X., Mohamed, D. K., Veksha, A., Handayani, M., Lisak, G. (2020). Carbon based copper(II) phthalocyanine catalysts for electrochemical CO2 reduction: Effect of carbon support on electrocatalytic activity. Carbon, 168, 245–253. doi: https://doi.org/10.1016/j.carbon.2020.06.066
- Ciptasari, N. I., Darsono, N., Handayani, M., Soedarsono, J. W. (2021). Synthesis of graphite oxide using hummers method: Oxidation time influence. AIP Conference Proceedings. doi: https://doi.org/10.1063/5.0061586
- Aliyev, E., Filiz, V., Khan, M. M., Lee, Y. J., Abetz, C., Abetz, V. (2019). Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials, 9 (8), 1180. doi: https://doi.org/10.3390/nano9081180
- Handayani, M., Sulistiyono, E., Rokhmanto, F., Darsono, N., Fransisca, P. L., Erryani, A., Wardono, J. T. (2019). Fabrication of Graphene Oxide/Calcium Carbonate/Chitosan Nanocomposite Film with Enhanced Mechanical Properties. IOP Conference Series: Materials Science and Engineering, 578 (1), 012073. doi: https://doi.org/10.1088/1757-899x/578/1/012073
- Handayani, M., Kepakisan, K. A. A., Anshori, I., Darsono, N., Nugraha T., Y. (2021). Graphene oxide based nanocomposite modified screen printed carbon electrode for qualitative cefixime detection. AIP Conference Proceedings. doi: https://doi.org/10.1063/5.0060625
- Zhang, L., Tan, Q., Kou, H., Wu, D., Zhang, W., Xiong, J. (2019). Highly Sensitive NH3 Wireless Sensor Based on Ag-RGO Composite Operated at Room-temperature. Scientific Reports, 9 (1). doi: https://doi.org/10.1038/s41598-019-46213-9
- Krisnandi, Y. K., Abdullah, I., Prabawanta, I. B. G., Handayani, M. (2020). In-situ hydrothermal synthesis of nickel nanoparticle/reduced graphene oxides as catalyst on CO2 methanation. AIP Conference Proceedings. doi: https://doi.org/10.1063/5.0007992
- Liu, G., Huang, L., Wang, Y., Tang, J., Wang, Y., Cheng, M. et al. (2017). Preparation of a graphene/silver hybrid membrane as a new nanofiltration membrane. RSC Adv., 7 (77), 49159–49165. doi: https://doi.org/10.1039/c7ra07904d
- Gurunathan, S., Han, J. W., Park, J. H., Kim, E., Choi, Y., Kwon, D., Kim, J. (2015). Reduced graphene oxide–silver nanoparticle nanocomposite: a potential anticancer nanotherapy. Int J Nanomedicine, 10 (1), 6257–6276. doi: https://doi.org/10.2147/ijn.s92449
- Ciotta, E., Prosposito, P., Tagliatesta, P., Lorecchio, C., Stella, L., Kaciulis, S. et al. (2018). Discriminating between Different Heavy Metal Ions with Fullerene-Derived Nanoparticles. Sensors, 18 (5), 1496. doi: https://doi.org/10.3390/s18051496
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Nurhayati Indah Ciptasari, Murni Handayani, Caesart Leonardo Kaharudin, Afif Akmal Afkauni, Adhi Dwi Hatmanto, Isa Anshori, Ahmad Maksum, Rini Riastuti, Johny Wahyuadi Soedarsono

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.









