Effect of the composition of emulsifiers and the dispersion medium on the properties of bases for semi-solid preparations
DOI:
https://doi.org/10.15587/2519-4852.2022.266001Keywords:
cetostearyl alcohol (CSA), propylene glycol (PG), basis, spin probe, rheological parameters, in vitro release testAbstract
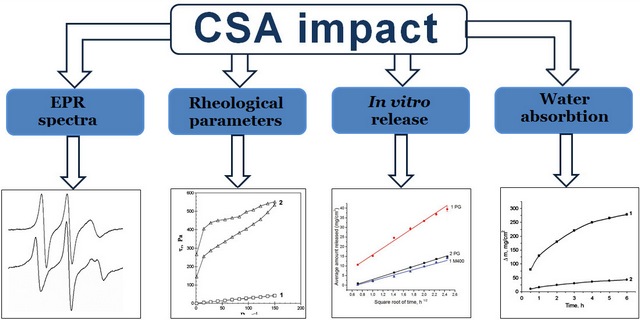
The aim. To study the effect of cetostearyl alcohol (CSA) on the rheological properties of bases with different dispersion media, the release of propylene glycol (PG) from them, and the ability of these bases to absorb water.
Materials and methods. Micelles of a non-ionic surfactant and its aggregates with CSA in a mixed solvent where the structure of water prevails, mixed solvent PG – macrogol 400 (M400) and hydrophilic bases-vehicles with different dispersion media were studied. The research was carried out by the spin probe method using a probe simulating a cationic surfactant and by rotational viscometry. The microstructure of the bases was studied by optical microscopy. The in vitro release test to study the release of PG and M400 from solutions and bases was performed using vertical diffusion chambers. The content of PG and M400 in the dialysate was determined by gas chromatography according to the validated analytical procedures. The absorption of water by solutions and bases was determined by dialysis through the membrane.
Results. CSA, which was the part of the bases together with surfactants in certain ratios, was a significant factor in increasing their rheological parameters, reducing the parameters of PG release during in vitro release tests, as well as reducing water absorption. The mechanisms of such influence are different for bases with different structures of the dispersion medium. In the bases, where the structure of water prevailed, lateral phase separation occurred in the supramolecular structures of surfactant and CSA with the formation of liquid domains of surfactant and solid domains of CSA, which contributed to the formation of coagulation structures. In the mixed non-aqueous solvent PG – M400, surfactant micelles and mixed aggregates of surfactant and CSA molecules were not formed; at 25 oC, surfactants and CSA became separate dispersed phases of suspensions, which contributed to the formation of gels. When CSA was added into an aqueous solution of poloxamer 338, PG, M400 and cationic surfactant, the flow behaviour changed, and the rheological parameters increased, which led to a decrease in the release rate and extended for PG and M400 as well as in the ability to absorb water. The rate and extent of PG release from the solution were greater compared to the M400 release.
Conclusions. The addition of CSA in combination with surfactants into the bases for semi-solid preparations is a significant factor for modifying their rheological parameters, the kinetics of PG release from them, and water absorption during experiments in vitro. The mechanisms of such an effect are different and depend on the composition and structure of the dispersion medium of the base
References
- Buckingham, R. (Ed.) (2020). Martindale: The Complete Drug Reference. London: Pharmaceutical Press, 4912.
- Ilić, T., Pantelić, I., Savić, S. (2021). The Implications of Regulatory Framework for Topical Semisolid Drug Products: From Critical Quality and Performance Attributes towards Establishing Bioequivalence. Pharmaceutics, 13 (5), 710. doi: https://doi.org/10.3390/pharmaceutics13050710
- Shanley, A. (2016). Topical Formulation: Moving from Art to Science. APIs, Excipients, and Manufacturing 2016 Supplement to Pharmaceutical Technology, 40 (9).
- Bezuglaya, E., Lyapunov, N., Lysokobylka, O., Liapunov, O., Klochkov, V., Grygorova, H., Liapunova, A. (2021). Interaction of surfactants with poloxamers 338 and its effect on some properties of cream base. ScienceRise: Pharmaceutical Science, 6 (34), 4–19. doi: https://doi.org/10.15587/2519-4852.2021.249312
- Wu, K., Yeoh, T., Hsieh, Y. L., Osborne, D. W.; Langley, N., Michniak-Kohn, B., Osborne, D. W. (Eds.) (2019). Quality Assesment of API in Topical Drug Products. The Role of Microstructure in Topical Drug Product Development. Cham: Springer, 36, 109–154. doi: http://doi.org/10.1007/978-3-030-17355-5_4
- Guideline on the Investigation of Bioequivalence (2010). CPMP/EWP/QWP/1401/98 Rev. 1 / Corr **. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf
- Raghavan, L., Brown, M., Michniak-Kohn, B., Sammeta, S.; Langley, N., Michniak-Kohn, B., Osborne, D. W. (Eds.) (2019). Quality Assesment of API in Topical Drug Products. The Role of Microstructure in Topical Drug Product Development. Springer: Cham, 36, 47–87. doi: http://doi.org/10.1007/978-3-030-17355-5_2
- SUPAC-SS. Guidance for Industry Nonsterile Semisolid Dosage Forms. Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation (1997). Rockville: Center for Drug Evaluation and Research (CDER). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ss-nonsterile-semisolid-dosage-forms-scale-and-post-approval-changes-chemistry-manufacturing
- Draft guideline on quality and equivalence of topical products (2018). CHMP/QWP/708282/2018. Available at: www.ema.europa.eu/en/quality-equivalence-topical-products
- The United States Pharmacopoeia, 41 – NF 36 (2018). The United States Pharmacopoeial Convention. Rockville. Available at: https://www.worldcat.org/title/united-states-pharmacopeia-2018-usp-41-the-national-formulary-nf-36/oclc/1013752699
- Miranda, M., Veloso, C., Brown, M., C. Pais, A. A. C., Cardoso, C., Vitorino, C. (2022). Topical bioequivalence: Experimental and regulatory considerations following formulation complexity. International Journal of Pharmaceutics, 620, 121705. doi: https://doi.org/10.1016/j.ijpharm.2022.121705
- Pleguezuelos-Villa, M., Merino-Sanjuán, M., Hernández, M. J., Nácher, A., Peris, D., Hidalgo, I. et. al. (2019). Relationship between rheological properties, in vitro release and in vivo equivalency of topical formulations of diclofenac. International Journal of Pharmaceutics, 572, 118755. doi: https://doi.org/10.1016/j.ijpharm.2019.118755
- The European Pharmacopoeia (2019). European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Strasbourg, 5224.
- Bezuglaya, E., Ivashchenko, H., Lyapunov, N., Zinchenko, I., Liapunova, A., Stolper, Y. et. al. (2021). Study of factors affecting the in vitro release of diclofenac sodium from hypromelose-based gels. ScienceRise: Pharmaceutical Science, 5 (33), 12–31. doi: https://doi.org/10.15587/2519-4852.2021.243040
- Sheskey, P. J., Hancock, B. C., Moss, G. P., Goldfarb, D. J. (Ed.) (2020). Handbook of Pharmaceutical Excipients. London: Pharm. Press, 1296.
- D’souza, A. A., Shegokar, R. (2016). Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opinion on Drug Delivery, 13 (9), 1257–1275. doi: https://doi.org/10.1080/17425247.2016.1182485
- Bodratti, A., Alexandridis, P. (2018). Formulation of poloxamers for drug delivery. Journal of Functional Biomaterials, 9 (1). doi: https://doi.org/10.3390/jfb9010011
- Mangas-Sanjuán, V., Pleguezuelos-Villa, M., Merino-Sanjuán, M., Hernández, M. J., Nácher, A., García-Arieta, A. et. al. (2019). Assessment of the Inter-Batch Variability of Microstructure Parameters in Topical Semisolids and Impact on the Demonstration of Equivalence. Pharmaceutics, 11 (10), 503. doi: https://doi.org/10.3390/pharmaceutics11100503
- Miranda, M., Pais, A. A. C. C., Cardoso, C., Vitorino, C. (2019). aQbD as a platform for IVRT method development – A regulatory oriented approach. International Journal of Pharmaceutics, 572, 118695. doi: https://doi.org/10.1016/j.ijpharm.2019.118695
- Miranda, M., Cova, T., Augusto, C., Pais, A. A. C. C., Cardoso, C., Vitorino, C. (2020). Diving into Batch-to-Batch Variability of Topical Products-a Regulatory Bottleneck. Pharmaceutical Research, 37 (11). doi: https://doi.org/10.1007/s11095-020-02911-y
- Xu, Z., Mangas-Sanjuán, V., Merino-Sanjuán, M., Merino, V., García-Arieta, A. (2020). Influence of Inter- and Intra-Batch Variability on the Sample Size Required for Demonstration of Equivalent Microstructure of Semisolid Dosage Forms. Pharmaceutics, 12 (12), 1159. doi: https://doi.org/10.3390/pharmaceutics12121159
- Benaouda, F., Brown, M. B., Ganguly, S., Jones, S. A., Martin, G. P. (2012). Discriminating the Molecular Identity and Function of Discrete Supramolecular Structures in Topical Pharmaceutical Formulations. Molecular Pharmaceutics, 9 (9), 2505–2512. doi: http://doi.org/10.1021/mp300127f
- Miron, D. S., Rădulescu, F. Ștefan, Voicu, V. A., Mînea, A., Cardot, J.-M., Shah, V. P. (2021). Rheological and in vitro release measurements of manufactured acyclovir 5% creams: confirming sensitivity of the in vitro release. Pharmaceutical Development and Technology, 26 (7), 779–787. doi: https://doi.org/10.1080/10837450.2021.1945625
- Benaouda, F., Jones, S. A., Martin, G. P., Brown, M. B. (2015). Localized Epidermal Drug Delivery Induced by Supramolecular Solvent Structuring. Molecular Pharmaceutics, 13 (1), 65–72. doi: https://doi.org/10.1021/acs.molpharmaceut.5b00499
- Carrer, V., Alonso, C., Pont, M., Zanuy, M., Córdoba, M., Espinosa, S. et. al. (2019). Effect of propylene glycol on the skin penetration of drugs. Archives of Dermatological Research, 312 (5), 337–352. doi: https://doi.org/10.1007/s00403-019-02017-5
- Zhang, W., Harty, B., Zheng, Y., Zhang, Z., Li, X., Wang, D., Kohane, D. S. (2021). Permeation of polyethylene glycols across the tympanic membrane. Giant, 6, 100057. doi: https://doi.org/10.1016/j.giant.2021.100057
- Laffleur, F., Pschick, S., Barthelmes, J., Hauptstein, S., Bernkop-Schnurch, A. (2018). Impact of Surfactants on Skin Penetration of Dexpanthenol. Current Drug Delivery, 15 (3), 351–356. doi: https://doi.org/10.2174/1567201814666170503142707
- Kováčik, A., Kopečná, M., Vávrová, K. (2020). Permeation enhancers in transdermal drug delivery: benefits and limitations. Expert Opinion on Drug Delivery, 17 (2), 145–155. doi: https://doi.org/10.1080/17425247.2020.1713087
- Liapunov, M. O., Ivanov, L. V., Bezuhla, O. P., Zhdanov, R. I., Tsymbal, L. V. (1992). Doslidzhennia ahrehativ poverkhnevo-aktyvnykh rechovyn (PAR) metodom spinovykh zondiv. Farmatsevtychnyi zhurnal, 5-6, 40–45.
- Derzhavna Farmakopeia Ukrainy. Tom 1 (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- Krasnoperova, A. P., Sytnik, O. Iu., Iukhno, G. D., Bezuglaia E. P., Liapunov, N. A., Krichevskii, A. O. (2002). Nekotorye zakonomernosti rastvoreniia i solvatatcii mikonazola v dvoinykh i troinykh smeshannykh rastvoriteliakh na osnove polietilenglikolia-400. Zhurnal prikladnoi khimii, 75 (12), 1972–1975.
- Berliner, L. (Ed.) (1979). Metod spinovykh metok. Teoriia i primenenie. Moscow: Mir, 635.
- Likhtenshtein, G. I. (1974). Metod spinovykh zondov v molekuliarnoi biologii, Moscow: Nauka, 256.
- Kuznetcov, A. N. (1976). Metod spinovogo zonda (Osnovy i primenenie). Moscow: Nauka, 210.
- Tiffner, K. I., Kanfer, I., Augustin, T., Raml, R., Raney, S. G., Sinner, F. (2018). A comprehensive approach to qualify and validate the essential parameters of an in vitro release test (IVRT) method for acyclovir cream, 5 %. International Journal of Pharmaceutics, 535 (1-2), 217–227. doi: https://doi.org/10.1016/j.ijpharm.2017.09.049
- Bezuglaya, E., Lyapunov, N., Bovtenko, V., Zinchenko, I., Stolper, Y. (2021). Study of pressurised metered dose inhalers for the purpose of standardization of quality attributes characterizing uniformity of dosing. ScienceRise: Pharmaceutical Science, 4 (32), 11–23. doi: https://doi.org/10.15587/2519-4852.2021.238294
- Note for Guidance on Validation of Analytical Procedures: Text and Methodology, Step 5 (1995). CPMP/ICH/381/95 (ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology).
- Ivanova, R., Alexandridis, P., & Lindman, B. (2001). Interaction of poloxamer block copolymers with cosolvents and surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 183-185, 41–53. doi: https://doi.org/10.1016/s0927-7757(01)00538-6
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Nikolay Lyapunov, Elena Bezuglaya, Anna Liapunova, Igor Zinchenko, Oleksii Liapunov, Oleksii Lysokobylka, Yurij Stolper

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







