Potential antioxidants of secondary metabolite isolates ethyl acetate fraction Coleus amboinicus Lour. Leaves.
DOI:
https://doi.org/10.15587/2519-4852.2022.266401Keywords:
Secondary metabolites, Coleus amboinicus Lour., DPPH, acetoxyhorminoneAbstract
The aim of the study was to isolate and characterize secondary metabolites that have the potential as antioxidants from the ethyl acetate fraction of the leaves of Coleus amboinicus, L. (C. amboinicus).
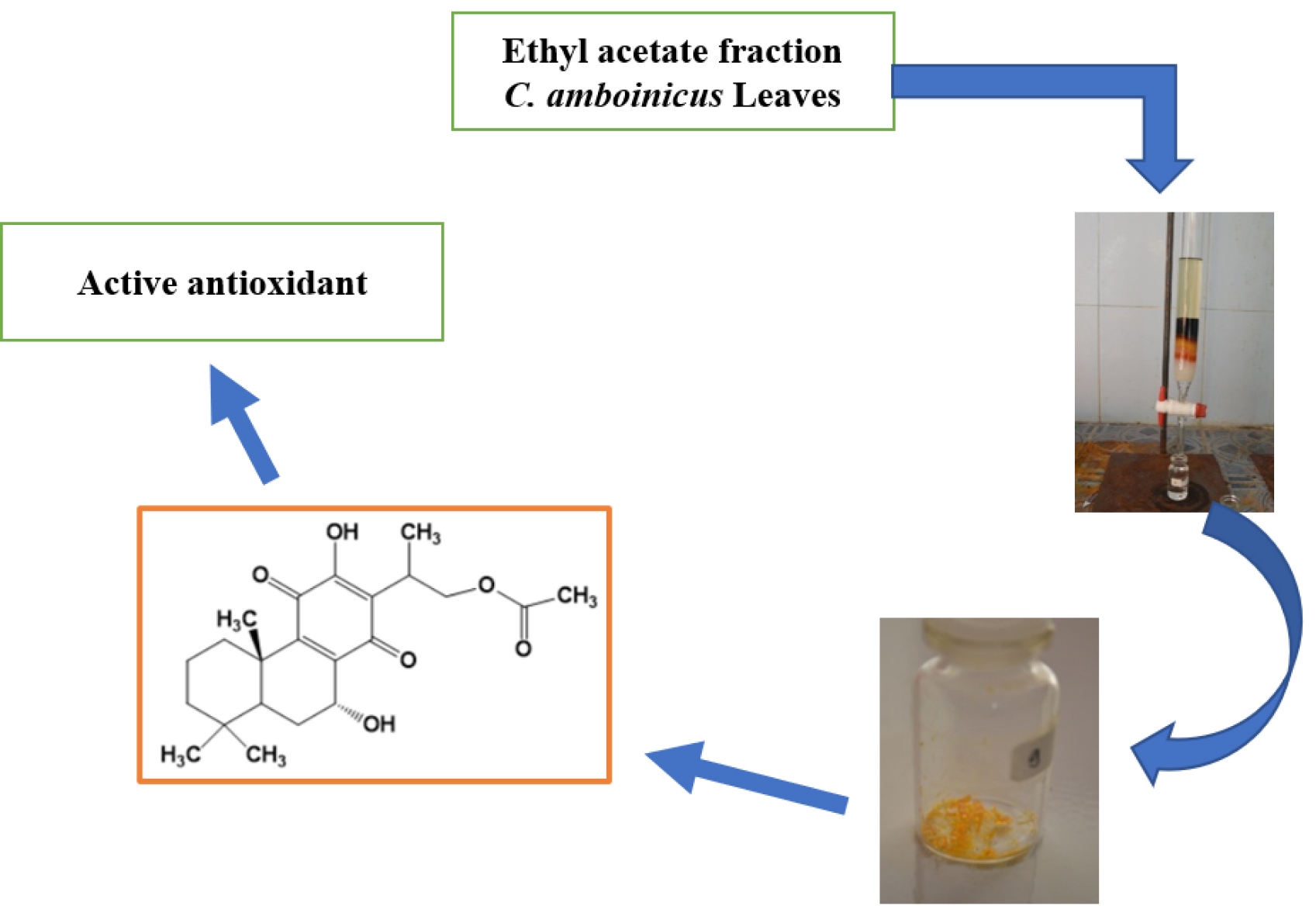
Materials and methods. Purification of the ethyl acetate fraction of C. amboinicus using gravity column chromatography with a stationary phase (silica gel, Merck) and a mobile phase with a solvent ratio of n-hexane (Merck) and ethyl acetate (Merck). Examining isolates includes physical (colour, shape, and melting point). Qualitative purity detection by TLC at 254 nm and 366 nm wavelengths. Structural analysis of metabolites with UV-Vis spectrometer (Spectronic 3000, Genesis 10, Japan), FT-IR(KBr) (Shimadzu IR Prestige-21, Japan), NMR spectrometer (JEOL spectrometer, Japan) operating at 500 MHz (1H-NMR) and 125 MHz (13C-NMR), and Shimadzu's GC-MS (QP-2010S Shimadzu, Japan) and determination of potential antioxidant activity using the DPPH method.
Results. The secondary metabolite compounds were isolated in the form of yellow crystals with a melting point of 232-233 °C and Rf values of 0.86 and 0.56, which TLC monitored at a solvent ratio of n-hexane and ethyl acetate 6:4 and 8:2. Spectronic analysis with a UV-Vis Spectrometer showed two electron absorbances, namely a wavelength of 210 nm indicating methanol solvent and 272 nm isolate. The absorbance of functional groups at wave numbers 3379 cm-1 (-OH; hydroxy), 2931 cm-1 (-CH; aliphatic), 1735 cm-1 (-C=O; carbonyl ketone), 1234 cm-1 (-CO-; methoxy) and 1643 cm-1 (-C=C-; alkene). GC-MS analysis obtained two absorbance peaks, (1) the first retention time of 6.658 minutes (3.95 %) and (2) the second retention time of 9.001 minutes (96.05 %). Structural analysis with 1H&13C-NMR showed 28 types of protons and 22 types of carbon. The antioxidant activity potential test showed an activity value (IC50) of 338.54 ppm.
Conclusion. The structure of the isolated secondary metabolite compound is 16-acetoxy-7α-hydroxyroyleanone (syn. 16-acetoxyhorminone) and has the potential as an antioxidant.
References
- Gupta, N., Verma, K., Nalla, S., Kulshreshtha, A., Lall, R., Prasad, S. (2020). Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules, 25 (22), 5390. doi: https://doi.org/10.3390/molecules25225390
- Mahantesh, S. P., Gangawane, A. K., Patil, C. S. (2021). Free Radicals, Antioxidants, Diseases and Phytomedicines in Human Health: Future Rerspects. World Research Journal of Medicinal & Aromatic Plants, 1 (1), 6–10.
- Sharma, G. N., Gupta, G., Sharma, P. (2018). A Comprehensive Review of Free Radicals, Antioxidants, and Their Relationship with Human Ailments. Critical Reviews in Eukaryotic Gene Expression, 28 (2), 139–154. doi: https://doi.org/10.1615/critreveukaryotgeneexpr.2018022258
- Pervin, M., Hasnat, Md., Lee, Y., Kim, D., Jo, J., Lim, B. (2014). Antioxidant Activity and Acetylcholinesterase Inhibition of Grape Skin Anthocyanin (GSA). Molecules, 19 (7), 9403–9418. doi: https://doi.org/10.3390/molecules19079403
- Jamshidi-kia, F., Wibowo, J. P., Elachouri, M., Masumi, R., Salehifard-Jouneghani, A. et al. (2020). Battle between plants as antioxidants with free radicals in human body. Journal of Herbmed Pharmacology, 9 (3), 191–199. doi: https://doi.org/10.34172/jhp.2020.25
- Amorati, R., Valgimigli, L. (2018). Methods To Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. Journal of Agricultural and Food Chemistry, 66 (13), 3324–3329. doi: https://doi.org/10.1021/acs.jafc.8b01079
- Lourenço, S. C., Moldão-Martins, M., Alves, V. D. (2019). Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules, 24 (22), 4132. doi: https://doi.org/10.3390/molecules24224132
- Turcov, D., Barna, S., Profire, L., Iacob, A.T., Lisa, G., Puitel, A. C. et al. (2022). Physico-Chemical Characterization of The Antioxidant Mixture Resveratrol-Ferulic Acid for Applications in Dermato-Cosmetic Products. FARMACIA. 70 (3), 410–416. doi: http://doi.org/10.31925/farmacia.2022.3.5
- Sirivibulkovit, K., Nouanthavong, S., Sameenoi, Y. (2018). Paper-based DPPH Assay for Antioxidant Activity Analysis. Analytical Sciences, 34 (7), 795–800. doi: https://doi.org/10.2116/analsci.18p014
- Ivanišová, E., Tokár, M., Mocko, K., Bojňanská, T, Mareček, J., Mendelová, A. (2013). Antioxidant Activity of Selected Plant Products. Journal of Microbiology Biotechnology and Food Sciences, 2 (1), 1692–1703.
- Gurning, K. (2020). Identication of Secondary Metabolic and Test of Activity Ethyl Acetate Fraction of bangun-Bangun (Coleus amboinicus Lour.) Leaves as Antioxidant. Biolink, 7 (1), 117–122. doi: http://doi.org/10.31289/biolink.v7i1.3732
- Khare, R. S., Banerjee, S., Kundu, K. (2011). Coleus Aromaticus Benth-A Nutritive Medicinal Plant of Potential Therapeutic Value. International Journal of Pharma and Bioscience and Technology, 2 (3), 488–500.
- Pillai, P. G., Suresh, P., Aggarwal, G., Doshi, G., Bhatia, V. (2011). Pharmacognostical Standardization and Toxicity Propile of the Methanolic Leaf Extract of Pectranthus amboinicus (Lour) Spreng. Journal of Applied Pharmaceutical Sciences, 1 (2), 75–81.
- Denes, T., Papp, N., Fogarasi, E., Marton, S. E., Varga, E. (2022). Phytochemical Investigation and Antioxidant Potential of Ononis Arvensis L. FARMACIA, 70 (3), 529–535. doi: http://doi.org/ 10.31925/farmacia.2022.3.20
- Gurning, K., Haryadi, W., Sastrohamidjojo, H. (2021). Isolation and Charactericzation of Antioxidant Compounds of Bangun-bangun (Coleus amboinicus, L.) Leaves from North Sumatera, Indonesia. Rasayan Journal Chemistry, 14 (1), 248–253. doi: http://doi.org/10.31788/RJC.2021.1416077
- Sinaga, S. P., Lumbangaol, D. A., Iksen, Situmorang, R. F. R., Gurning, K. (2022). Determination of Phenolic, Flavonoid Content, Antioxidant and Antibacterial Activities of Seri (Muntingia calabura L.) Leaves Ethanol Extract from North Sumatera, Indonesia. Rasayan Journal Chemistry, 15 (2), 1534–1538. doi: http://doi.org/10.31788/RJC.2022.1526730
- William, D.H., Ian, F. (2008). Spectroscopic Methods in Organic Chemistry. London: The Mcbraw-Hill Companies. doi: https://doi.org/10.1055/b-0035-108183
- Žuvela, P., Skoczylas, M., Jay Liu, J., Ba̧czek, T., Kaliszan, R., Wong, M. W., Buszewski, B. (2019). Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chemical Reviews, 119 (6), 3674–3729. doi: https://doi.org/10.1021/acs.chemrev.8b00246
- Jurkaninova, S., Kubinova, R., Nejezchlebova, M., Gazdova, M., Hanakova,Z., Dall’Acqua, S. (2018). Anti-MRSA activity of abietane diterpenes from Coleus blumei Benth. Natural Product Research, 35 (18), 3033–3039. doi: https://doi.org/10.1080/14786419.2019.1686371
- Gaborova, M., Smejkal, K., Kubinova, R. (2022). Abietane Diterpenes of the Genus Plectranthus sensu lato. Molecules, 27 (1), 1–64. doi: https://doi.org/10.3390/molecules27010166
- Garcia, C., Silva, C. O., Monteiro, C. M., Nicolai, M., Viana, A., Andrade, J. M. et al. (2018). Anticancer properties of the abietane diterpene 6,7-dehydroroyleanone obtained by optimized extraction. Future Medicinal Chemistry, 10 (10), 1177–1189. doi: https://doi.org/10.4155/fmc-2017-0239
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Kasta Gurning, Winarto Haryadi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







