Establishment of technologically feasible modes of electrocoagulation purification of wastewater from nickel ions
DOI:
https://doi.org/10.15587/2706-5448.2023.278006Keywords:
wastewater purification, electrochemical purification, heavy metals, Nickel compounds, electrocoagulation, aluminum electrodesAbstract
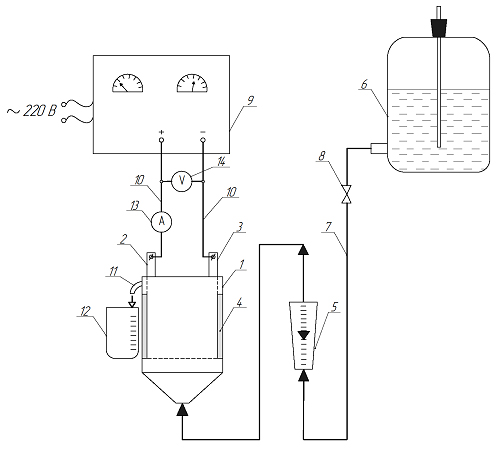
The object of research is the technology of electrochemical purification of wastewater from heavy metal ions. The work, in particular, is devoted to the purification of effluents with a low concentration of the Nickel ion. The main task of the experimental research was to select the material of the electrodes and the mode of electrochemical dissolution of the anodes, under which the efficiency of wastewater purification will be maximum, as well as to confirm the practical possibility of deep purification of the specified wastewater to the standards that correspond to the maximum permissible concentrations (MPCNi=0.5 mg/dm3).
It was established that in the process of electrocoagulation wastewater purification there is an induction period (10 min), during which the coagulant accumulates in the electrolyzer and the degree of purification increases sharply. As a result of an increase in the current density from 10 A/m2 to 20 A/m2, the degree of purification on iron electrodes increased from 60 to 84% for a process duration of 20 minutes. This is explained by the intensification of the anodic dissolution of the metal and the increase in the concentration of Fe(OH)3. Increasing the current density to 30 A/m2 practically does not affect the degree of purification, which is explained by the phenomenon of polarization of the anodes and is confirmed by the increase in the process voltage from 2.40 V (10 A/m2) to 12.59 V (30 A/m2). Therefore, it is impractical to increase the current density in the future. For iron anodes, it was not possible to achieve the required degree of purification (≥98.3 %), the maximum degree of purification did not exceed 85 %, and the content of Ni2+ ions in purified water exceeds the MPC by an order of magnitude.
It was experimentally established that it is advisable to use aluminum electrodes for the process of electrocoagulation purification of wastewater from Nickel ions. At a current density of 20 A/m 2 and process duration of 40 minutes, the concentration of Ni2+ ions did not exceed the MPC of Ni. When using aluminum electrodes, an increase in the current density from 15 A/m2 to 20 A/m2 does not lead to polarization of the electrodes, and the process in both cases takes place at a steady state at a voltage of ~6.7 V. The technologically appropriate operating mode of the electrolyzer is chosen: aluminum electrodes at an anodic current density 20 A/m2 and the duration of the purification process – ≥40 min. The obtained results can find practical use in the design of waste water purification systems of galvanic industries.
References
- Elbehiry, F., Alshaal, T., Elhawat, N., Elbasiouny, H. (2021). Environmental-Friendly and Cost-Effective Agricultural Wastes for Heavy Metals and Toxicants Removal from Wastewater. Cost-Efficient Wastewater Treatment Technologies, 107–127. doi: https://doi.org/10.1007/698_2021_786
- Ensure availability and sustainable management of water and sanitation for all (2020). Sustainable Development Goals 6. United Nations. Available at: https://sustainabledevelopment.un.org/sdg6
- Ferrante, M., Conti, G. O., Rasic-Milutinovic, Z., Jovanovic, D. (2013). Health effects of metals and related substances in drinking water. IWA Publishing.
- Nickel in drinking water: Background document for development of WHO guidelines for drinking-water quality (WHO/HEP/ECH/WSH/2021.6) (2021). World Health Organization, 36. Available at: https://apps.who.int/iris/handle/10665/350934
- Rajoria, S., Vashishtha, M., Sangal, V. K. (2022). Treatment of electroplating industry wastewater: a review on the various techniques. Environmental Science and Pollution Research, 29 (48), 72196–72246. doi: https://doi.org/10.1007/s11356-022-18643-y
- Azimi, A., Azari, A., Rezakazemi, M., Ansarpour, M. (2017). Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Reviews, 4 (1), 37–59. doi: https://doi.org/10.1002/cben.201600010
- Rajoria, S., Vashishtha, M., Sangal, V. K. (2021). Review on the treatment of electroplating industry wastewater by electrochemical methods. Materials Today: Proceedings, 47, 1472–1479. doi: https://doi.org/10.1016/j.matpr.2021.04.165
- Fu, R., Zhang, P.-S., Jiang, Y.-X., Sun, L., Sun, X.-H. (2023). Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere, 311, 136993. doi: https://doi.org/10.1016/j.chemosphere.2022.136993
- Kazeminezhad, I., Mosivand, S. (2017). Elimination of copper and nickel from wastewater by electrooxidation method. Journal of Magnetism and Magnetic Materials, 422, 84–92. doi: https://doi.org/10.1016/j.jmmm.2016.08.049
- Guan, W., Tian, S., Cao, D., Chen, Y., Zhao, X. (2017). Electrooxidation of nickel-ammonia complexes and simultaneous electrodeposition recovery of nickel from practical nickel-electroplating rinse wastewater. Electrochimica Acta, 246, 1230–1236. doi: https://doi.org/10.1016/j.electacta.2017.06.121
- Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology, 38 (1), 11–41. doi: https://doi.org/10.1016/j.seppur.2003.10.006
- Hakizimana, J. N., Gourich, B., Chafi, M., Stiriba, Y., Vial, C., Drogui, P., Naja, J. (2017). Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination, 404, 1–21. doi: https://doi.org/10.1016/j.desal.2016.10.011
- Yakovlev, S. V., Krasnoborodko, I. G., Rogov, V. M. (1987). Tehnologiya elektrohimicheskoy ochistki vodyi. Leningrad: Stroyizdat, 158.
- Comninellis, C., Chen, G. (Eds.) (2010). Electrochemistry for the Environment. New York: Springer. doi: https://doi.org/10.1007/978-0-387-68318-8
- Kuznetsova, T. A., Pestov, N. A., Revin, V. V. (2020). Study of the adsorption properties of plant cellulose with respect to nickel ions. Chemistry of Plant Raw Material, 2, 307–314. doi: https://doi.org/10.14258/jcprm.2020026573
- Kalymon, Y. A., Znak, Z. O., Helesh, A. B., Savchuk, L. V. (2018). Investigation of the absorption process of air oxygen in a device with a continuous bubbling layer. Voprosy Khimii i Khimicheskoi Tekhnologii, 5, 102–110.
- Kalymon, Ya., Helesh, A., Slyuzar, A., Kurylets, O. (2022). Choice of mass exchange apparatus for groundwater deironing. Chemistry, Technology and Application of Substances, 5 (1), 29–35. doi: https://doi.org/10.23939/ctas2022.01.029
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Andriy Helesh, Petro Mudrynets, Yaroslav Kalymon, Diana Kindzera, Vira Hnativ

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.








