Clinical and neurological features of different variants of peripheral polyneuropathy in patients with type 2 diabetes mellitus
DOI:
https://doi.org/10.15587/2519-4798.2024.324683Keywords:
diabetes mellitus, polyneuropathy, neurological examination, electroneuromyographic examination, pain syndrome, sensitivity, symmetryAbstract
The aim: to determine the clinical and neurological features of polyneuropathies depending on the pathomorphological type of peripheral nerve damage in patients with type 2 diabetes mellitus (DM).
Materials and methods: We examined 96 patients who were being treated in the endocrinology department of the University Hospital of KhNMU, with a diagnosis of type 2 DM and a complication - peripheral polyneuropathy (PNP). All patients completed a general clinical and neurological examination, assessment of the severity of polyneuropathic syndrome using specialized scales, and electroneuromyographic examination to assess the morpho-functional type of peripheral nerve damage.
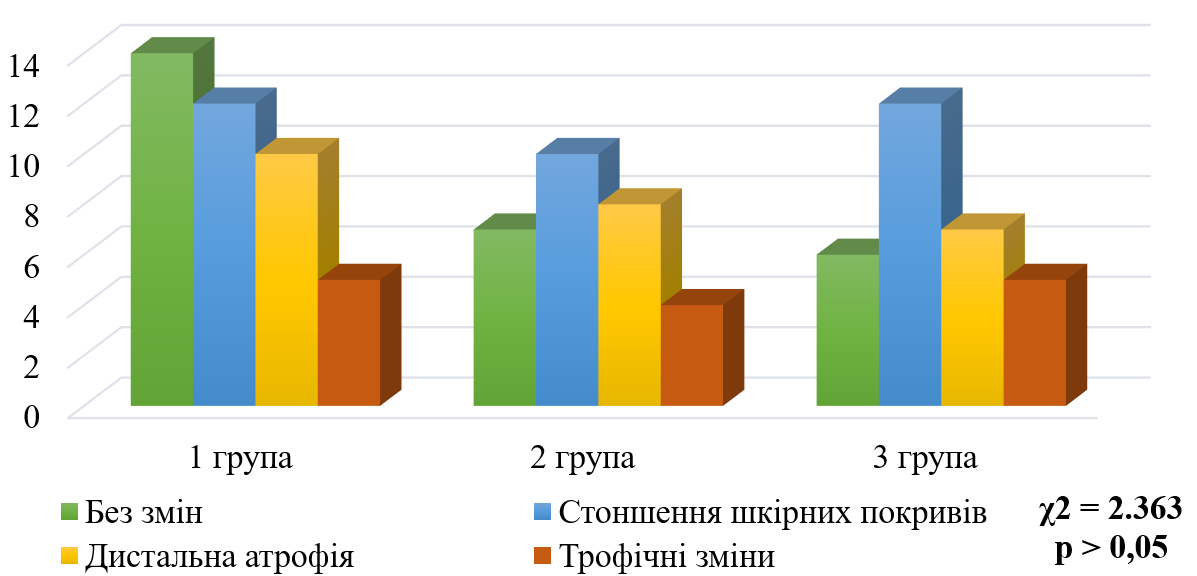
Results: All patients were divided into three groups according to the results of electroneuromyographic examination: group 1 - axonal type (n = 37); group 2 - demyelinating type (n = 29); group 3 - mixed (axonal-demyelinating) type (n = 30). Further, during the study, depending on the morphofunctional type of peripheral nerve damage, the clinical and neurological status of patients and the results of valid scales (TSS, NIS LL, MNSI, The pain DETECT questionnaire, the VAS analogue scale) were analyzed. The symmetry of neurological symptoms and clinical and neurological status disorders in patients with different types of peripheral neuropathy were also investigated.
Conclusions: The study showed that patients with axonal PNP in type 2 DM are more characterized by subjective neurological symptoms and reduced reflexes. In patients with a demyelinating type of PNP, impaired vibration sensitivity, decreased muscle strength in the extremities, and increased tendon reflexes of the lower extremities predominate. Patients with mixed types of PNP complain more of symptoms characteristic of axonal damage, and impaired vibration sensitivity and decreased muscle strength are also observed
References
- Panou, T., Gouveri, E., Papazoglou, D., Papanas, N. (2024). The role of novel inflammation-associated biomarkers in diabetic peripheral neuropathy. Metabolism Open, 24, 100328. https://doi.org/10.1016/j.metop.2024.100328
- Zhang, D., Huang, Y., Guan, Y., Zhang, X., Pan, P., Yan, X. et al. (2024). Characterization of changes in the resting-state intrinsic network in patients with diabetic peripheral neuropathy. Scientific Reports, 14 (1). https://doi.org/10.1038/s41598-024-80216-5
- Yang, Z., Zhao, S., Lv, Y., Xiang, L., Zhang, X., Feng, Z. et al. (2024). A New Quantitative Neuropad for Early Diagnosis of Diabetic Peripheral Neuropathy. Diabetes/Metabolism Research and Reviews, 40 (8). https://doi.org/10.1002/dmrr.70010
- Hsieh, P., Ro, L., Chu, C., Liao, M., Chang, H., Kuo, H. (2024). Relationship between nerve ultrasonography image and electrophysiology in diabetic polyneuropathy. Journal of Diabetes Investigation, 16 (2), 257–264. Portico. https://doi.org/10.1111/jdi.14353
- Mendoza-Romo, M. Á., Ortiz-Martinez, A. Y., Fabela-Mendoza, K., García-Hernández, J. A., Acuña-López, M. A., Miramontes-Zapata, M. (2021). Manifestaciones clínicas y alteraciones electroneuromiográficas en pacientes con diabetes tipo 2 y polineuropatía Revista Médica del Instituto Mexicano del Seguro Social, 59 (3), 224–232.
- Singh, A., Kukreti, R., Saso, L., Kukreti, S. (2022). Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules, 27 (3), 950. https://doi.org/10.3390/molecules27030950
- Poitras, T. M., Munchrath, E., Zochodne, D. W. (2021). Neurobiological Opportunities in Diabetic Polyneuropathy. Neurotherapeutics, 18 (4), 2303–2323. https://doi.org/10.1007/s13311-021-01138-y
- Hernyák, M., Tóth, L. I., Csiha, S., Molnár, Á., Lőrincz, H., Paragh, G. et al. (2024). Kallistatin as a Potential Marker of Therapeutic Response During Alpha-Lipoic Acid Treatment in Diabetic Patients with Sensorimotor Polyneuropathy. International Journal of Molecular Sciences, 25 (24), 13276. https://doi.org/10.3390/ijms252413276
- Han, G., Hu, K., Luo, T., Wang, W., Zhang, D., Ouyang, L. et al. (2025). Research progress of non-coding RNA regulating the role of PANoptosis in diabetes mellitus and its complications. Apoptosis. https://doi.org/10.1007/s10495-024-02066-w
- Souayah, N., Chong, Z. Z., Patel, T., Nasar, A., Pahwa, A., W Sander, H. (2024). Regression equation analysis enhances detection of conduction slowing beyond axonal loss in diabetic neuropathy. Heliyon, 10 (21), e39712. https://doi.org/10.1016/j.heliyon.2024.e39712
- Abd Razak, N. H., Idris, J., Hassan, N. H., Zaini, F., Muhamad, N., Daud, M. F. (2024). Unveiling the Role of Schwann Cell Plasticity in the Pathogenesis of Diabetic Peripheral Neuropathy. International Journal of Molecular Sciences, 25 (19), 10785. https://doi.org/10.3390/ijms251910785
- Zhang, X., Zhong, G., Jiang, C., Ha, X., Yang, Q., Wu, H. (2024). Exploring the potential anti-diabetic peripheral neuropathy mechanisms of Huangqi Guizhi Wuwu Decoction by network pharmacology and molecular docking. Metabolic Brain Disease, 40(1). https://doi.org/10.1007/s11011-024-01474-w
- Ziegler, D., Hanefeld, M., Ruhnau, K. J., Meissner, H. P., Lobisch, M., Schütte, K., Gries, F. A. (1995). Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant α‐lipoicacido. Diabetologia, 38 (12), 1425–1433. https://doi.org/10.1007/bf00400603
- Bril, V. (1999). NIS-LL: The Primary Measurement Scale for Clinical Trial Endpoints in Diabetic Peripheral Neuropathy. European Neurology, 41(Suppl. 1), 8–13. https://doi.org/10.1159/000052074
- Moghtaderi, A., Bakhshipour, A., Rashidi, H. (2006). Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clinical Neurology and Neurosurgery, 108 (5), 477–481. https://doi.org/10.1016/j.clineuro.2005.08.003
- Freynhagen, R., Baron, R., Gockel, U., Tölle, T. R. (2006). painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Current Medical Research and Opinion, 22 (10), 1911–1920. https://doi.org/10.1185/030079906x132488
- Vaeggemose, M., Pham, M., Ringgaard, S., Tankisi, H., Ejskjaer, N., Heiland, S. et al. (2017). Magnetic Resonance Neurography Visualizes Abnormalities in Sciatic and Tibial Nerves in Patients With Type 1 Diabetes and Neuropathy. Diabetes, 66 (7), 1779–1788. https://doi.org/10.2337/db16-1049
- Sierra-Silvestre, E., Smith, R. E., Andrade, R. J., Kennedy, B., Coppieters, M. W. (2024). Microstructural changes in the median and ulnar nerve in people with and without diabetic neuropathy in their hands: A cross-sectional diffusion MRI study. European Journal of Radiology, 181, 111721. https://doi.org/10.1016/j.ejrad.2024.111721
- Chang, M. C., Yang, S. (2023). Diabetic peripheral neuropathy essentials: a narrative review. Annals of Palliative Medicine, 12 (2), 390–398. https://doi.org/10.21037/apm-22-693
- Ferraz de Oliveira, I., Correia, I., Urzal, J., Cruz, S., Aldomiro, F. (2022). Chronic Inflammatory Demyelinating Polyradiculoneuropathy and Diabetes: A Case Report. Cureus, 14 (9), e29390. https://doi.org/10.7759/cureus.29390
- Li, J., Niu, B., Wang, X., Hu, H., Cao, B. (2017). A case report of hereditary neuropathy with liability to pressure palsies accompanied by type 2 diabetes mellitus and psoriasis. Medicine, 96 (19), e6922. https://doi.org/10.1097/md.0000000000006922
- Ettinger, L. R., Boucher, A., Simonovich, E. (2018). Patients with type 2 diabetes demonstrate proprioceptive deficit in the knee. World Journal of Diabetes, 9 (3), 59–65. https://doi.org/10.4239/wjd.v9.i3.59
- Maras, O., Dulgeroglu, D., Cakci, A. (2021). Ankle Proprioception in Patients with Type 2 Diabetes Mellitus. Journal of the American Podiatric Medical Association, 111 (4). https://doi.org/10.7547/18-178
- Miyashita, A., Kobayashi, M., Yokota, T., Zochodne, D. (2023). Diabetic Polyneuropathy: New Strategies to Target Sensory Neurons in Dorsal Root Ganglia. International Journal of Molecular Sciences, 24 (6), 5977. https://doi.org/10.3390/ijms24065977
- Stępień, J., Pastuszak, Ż. (2023). Distal symmetrical polyneuropathy in diabetes mellitus patients: Proposition of a new scoring system based on electroneurography findings. Advances in Clinical and Experimental Medicine, 33 (4), 379–385. https://doi.org/10.17219/acem/168504
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Daryna Sushetska, Olena Tovazhnyanska

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.








