Theoretical and experimental justification of the method for determining the parameters of the moment of gas hydrates mass crystallization
DOI:
https://doi.org/10.15587/2706-5448.2023.274183Keywords:
gas hydrates, phase transitions, mass crystallization, interphase contact, induction period of hydrate formation, gas bubbleAbstract
The processes of oil and gas production – extraction, preparation, storage and transportation of oil, gas and condensate – are accompanied by risks of man-made hydrate formation. Such man-made gas hydrates cause serious problems for the oil and gas production industry. Oil and gas companies bear significant material costs in connection with the prevention of these processes. For prevent or eliminate it in each specific case, it is necessary to understand the physics of processes and parameters of hydrate formation. Therefore, establishing the peculiarities of the kinetics and thermobaric parameters of the hydrate formation process is an urgent problem. Thus, the object for research is the parameters of the beginning of mass gas hydrates crystallization in reservoir systems. At the same time, the most reliable results can be obtained in the process of laboratory monitoring of processes in reservoir systems and technological equipment directly at industrial facilities.
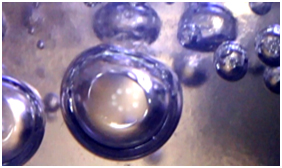
The process of hydrate formation at the phase boundary is manifested by the formation of a thin hydrate layer in the form of a film. In the course of experimental studies, it was established that this process is visually fixed by the transformation of the mirror surface of the phase boundary into a matte one. The distortion effect of the interphase boundary is explained by the formation, growth, massive and chaotic accumulation of gas hydrate microcrystals at this boundary. In the work, based on the results of theoretical and experimental studies, the methodology for operational laboratory determination of parameters of mass gas hydrate crystallization is substantiated. The essence of the technique is to establish the parameters for the moment of mass gas hydrates crystallization based on the fixation of the optical distortion effect of the reflection of the light source on the mirror of the liquid-gas interphase surface. The results of empirical studies are based on optical phenomena observed at the interfacial surface of the gas hydrate layer and gas. They were studied using microscopy, fixation and image processing methods. The main experiments result was the information recorded by the optical system and obtained after fixing the pressure and temperature.
The technique can be used to establish and operationally control the moment of mass gas hydrates crystallization directly at the objects of the oil and gas industry (during the implementation of technological processes). This will make it possible to effectively prevent clogging of technological equipment with the solid gas hydrate phase, as well as to prevent overuse of hydrate formation inhibitors. At the same time, the only limitation of the application for this technique may be the low light permeability of the aqueous solution as part of the formation system.
References
- Sloan, E. D. (2003). Fundamental principles and applications of natural gas hydrates. Nature, 426 (6964), 353–363. doi: https://doi.org/10.1038/nature02135
- Sloan, E. D. (1998). Clathrate hydrates of natural gases. New York: Marcel Dekker, 705.
- Kinnari, K., Hundseid, J., Li, X., Askvik, K. M. (2014). Hydrate Management in Practice. Journal of Chemical & Engineering Data, 60 (2), 437–446. doi: https://doi.org/10.1021/je500783u
- Zain, Z. M., Yang, J., Tohidi, B., Cripps, A., Hunt, A. (2005). Hydrate Monitoring and Warning System: A New Approach for Reducing Gas Hydrate Risks. SPE Europec/EAGE Annual Conference. Madrid. doi: https://doi.org/10.2118/94340-MS
- Kawasaki, T., Kikuchi, K., Terasaki, D., Okui, T., Miyata, K., Hirayama, H., Masaru, I. (2002). Composition of Guests in Hydrates from Gas Mixture. Proceedings of the fourth International Conference on Gas Hydrates: May 19-23, 2002, Symposia, Yokohama, Japan, 2, 488. Available at: https://ndlonline.ndl.go.jp/#!/detail/R300000001-I000003587697-00
- Tohidi, B., Anderson, R., Chapoy, A., Yang, J., Burgass, R. W. (2012). Do We Have New Solutions to the Old Problem of Gas Hydrates? Energy & Fuels, 26 (7), 4053–4058. doi: https://doi.org/10.1021/ef3002179
- Tohidi, B., Chapoy, A., Yang, J., Ahmadloo, F., Valko, I., Zain, Z. M. (2008). Developing Hydrate Monitoring and Early Warning Systems. Waves of Change. Houston, 1, 515–523. Available at: http://toc.proceedings.com/02832webtoc.pdf
- Sloan, E. D., Koh, C. A. (2008). Clathrate Hydrates of Natural Gases. CRC Press, 455.
- Turner, D. J. (2005) Clathrate Hydrate Formation in Water-in-oil Dispersions. Colorado School of Mines, Golden. Available at: http://hdl.handle.net/11124/78538
- Ohmura, R., Ogawa, M., Yasuoka, K., Mori, Y. H. (2003). Statistical Study of Clathrate-Hydrate Nucleation in a Water/Hydrochlorofluorocarbon System: Search for the Nature of the «Memory Effect». The Journal of Physical Chemistry B, 107 (22), 5289–5293. doi: https://doi.org/10.1021/jp027094e
- Parent, J. S., Bishnoi, P. R. (1996). Investigations into the nucleation behaviour of methane gas hydrates. Chemical Engineering Communications, 144 (1), 51–64. doi: https://doi.org/10.1080/00986449608936444
- Mchedlov-Petrosian, M. O., Lebid, V. I., Hlazkov, O. M., Lebid, O. V. (2012). Koloidna khimiia. Kharkiv: KhNU imeni V. N. Karazina, 500.
- Kostrzhytskyi, A. I., Kalinkov, O. Yu., Tishchenko, V. M., Berehova, O. M. (2008). Fizychna ta koloidna khimiia. Kyiv: Tsentr uchbovoi literatury, 496.
- Sloan, E., Koh, C., Sum, A. (2010). Natural gas hydrates in flow assurance. Elsevier, Gulf Professional Publishing, 224. Available at: https://www.elsevier.com/books/natural-gas-hydrates-in-flow-assurance/koh/978-1-85617-945-4
- Choukroun, M., Grasset, O., Tobie, G., Sotin, C. (2010). Stability of methane clathrate hydrates under pressure: Influence on outgassing processes of methane on Titan. Icarus, 205 (2), 581–593. doi: https://doi.org/10.1016/j.icarus.2009.08.011
- Pedchenko, N., Vynnykov, Y., Pedchenko, L., Pedchenko, M. (2021). Method for determining the starting moment of hydrate formation on the basis of optical effects. E3S Web of Conferences, 230, 01003. doi: https://doi.org/10.1051/e3sconf/202123001003
- Ostrovskii, G. M. (2000). Prikladnaia mekhanika neodnorodnykh sred. Saint-Petersburg: Nauka, 359.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Larysa Pedchenko, Mykhailo Pedchenko, Angela Yelchenko-Lobovska

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







