Investigation of energy efficiency of hydrogen production in alkaline electrolysers
DOI:
https://doi.org/10.15587/2706-5448.2023.290309Keywords:
electrolysis, electrolyzer, hydrogen, environmentally friendly energy production, renewable energy sources, alkaline electrolyteAbstract
The object of research is the energy efficiency of the electrolysis process in electrolyzers with alkaline electrolyte electrical parameters. The existing problem consists in obtaining the energy efficiency of the process in an electrolyzer with an alkaline electrolyte of more than 65 %.
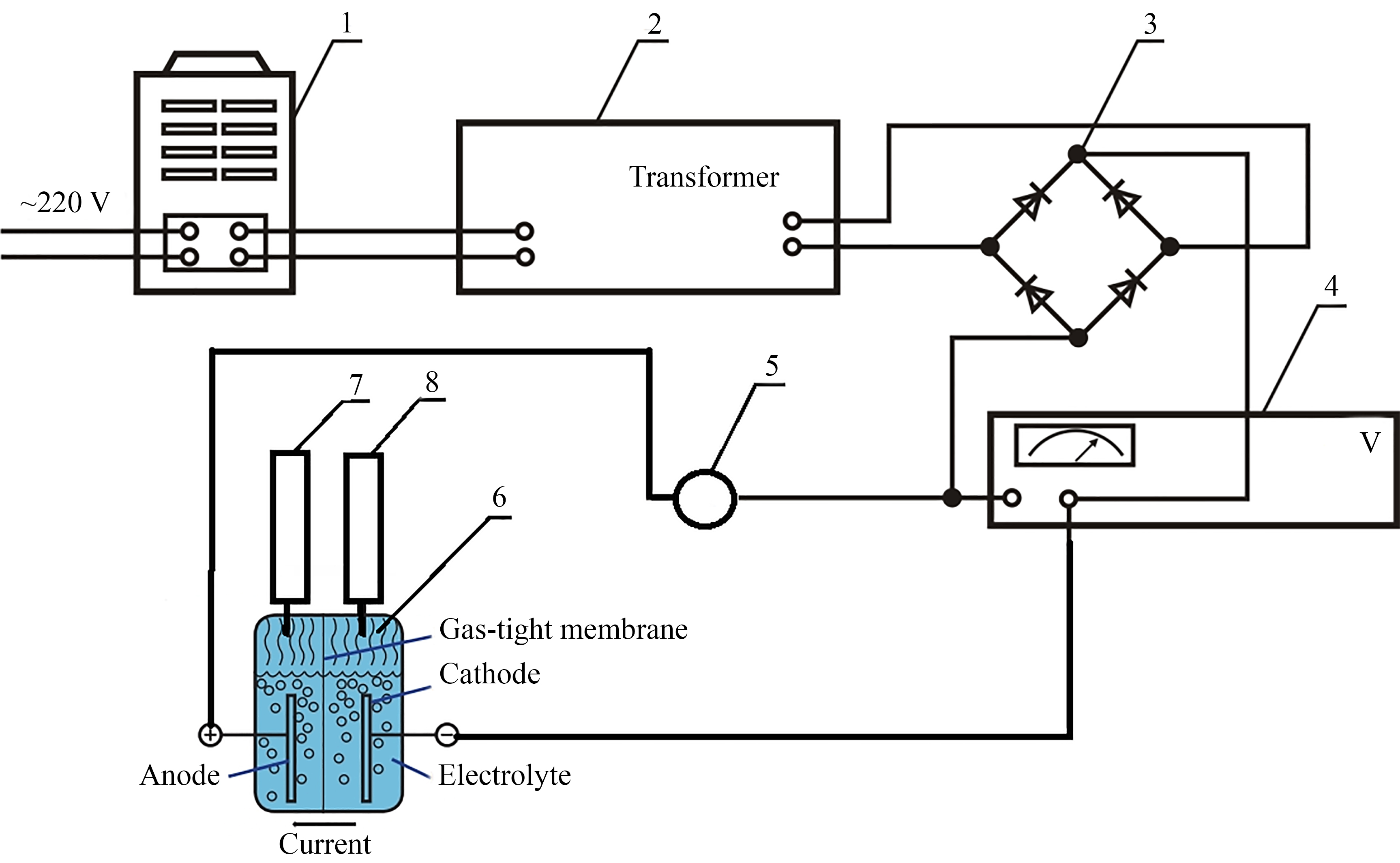
To solve this problem, it is proposed to manufacture an electrolyzer with metal electrodes made of stainless steel and separated from each other by a gas-tight membrane (Bologna cloth) to separate hydrogen and oxygen gases. To establish the energy efficiency characteristics, an experimental installation was made, and the necessary measuring equipment was also used. In the course of the work, a research methodology was developed and the necessary calculation of the measured values was carried out. As a result, the influence of the operating voltage on the consumption of the current flowing through the electrodes of the electrolyzer and the power consumed by it was revealed, the values of which increase with the increase of the operating voltage. It was established that the energy efficiency of the process in electrolyzers with an alkaline electrolyte decreases with an increase in the operating voltage. At operating voltages of 3 V, 4 V, and 5 V, the energy efficiency is 85.7 %, 77 %, and 70 %, respectively. The proposed technique involves conducting experimental studies directly on a functioning electrolyzer.
The practical implementation of the use of a gas-tight membrane (Bologna fabric) can help reduce the cost of manufacturing an electrolyzer. Therefore, the presented research will be useful for the industrial production of hydrogen.
References
- Lewis, N. S., Nocera, D. G. (2006). Powering the planet: Chemical challenges in solar energy utilization. Proceedings of the National Academy of Sciences, 103 (43), 15729–15735. doi: https://doi.org/10.1073/pnas.0603395103
- Dunikov, D. O. (2017). Vodorodnye energeticheskie tekhnologii. Materialy seminara laboratorii VET OIVT RAN. Moscow: OIVT RAN, 1, 190.
- Wirkert, F. J., Roth, J., Jagalski, S., Neuhaus, P., Rost, U., Brodmann, M. (2020). A modular design approach for PEM electrolyser systems with homogeneous operation conditions and highly efficient heat management. International Journal of Hydrogen Energy, 45 (2), 1226–1235. doi: https://doi.org/10.1016/j.ijhydene.2019.03.185
- Chang, W. J., Lee, K.-H., Ha, H., Jin, K., Kim, G., Hwang, S.-T. et al. (2017). Design Principle and Loss Engineering for Photovoltaic–Electrolysis Cell System. ACS Omega, 2 (3), 1009–1018. doi: https://doi.org/10.1021/acsomega.7b00012
- Kuznetcov, N. P., Lysenko, O. V., Chebanov, A. B. (2019). Electricity Consumption Model for Energy Systems of Ukraine at Various Levels of Locality. Problemele Energeticii Regionale, 3 (44), 31–42. doi: https://doi.org/10.5281/zenodo.3562195
- Gahleitner, G. (2013). Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. International Journal of Hydrogen Energy, 38 (5), 2039–2061. doi: https://doi.org/10.1016/j.ijhydene.2012.12.010
- Grätzel, M. (2001). Photoelectrochemical cells. Nature, 414 (6861), 338–344. doi: https://doi.org/10.1038/35104607
- Cox, C. R., Lee, J. Z., Nocera, D. G., Buonassisi, T. (2014). Ten-percent solar-to-fuel conversion with nonprecious materials. Proceedings of the National Academy of Sciences, 111 (39), 14057–14061. doi: https://doi.org/10.1073/pnas.1414290111
- Sytniuk, H. O., Nochnichenko, I. V., Kostiuk, D. V., Myronchuk, V. S. (2018). Elektroliz yak aktualnyi sposib otrymannia alternatyvnoho palyva vodniu. Prykladna heometriia, dyzain, ob’iekty intelektualnoi vlasnosti ta innovatsiina diialnist studentiv ta molodykh vchenykh. Kyiv, 115–119.
- Yakymenko, L. M., Motilievska, I. D., Tkachek, Z. O. (1970). Elektroliz vody. Moscow: Vyd. Khimiia, 264.
- Peharz, G., Dimroth, F., Wittstadt, U. (2007). Solar hydrogen production by water splitting with a conversion efficiency of 18 %. International Journal of Hydrogen Energy, 32 (15), 3248–3252. doi: https://doi.org/10.1016/j.ijhydene.2007.04.036
- Shevchenko, A. A., Zipunnikov, M. M., Kotenko, А. L., Chorna, N. A. (2020). Investigation of the Electrolysis Process of Obtaining Hydrogen and Oxygen with Serial and Parallel Connection of Electrons. Journal of Mechanical Engineering, 23 (4), 63–71. doi: https://doi.org/10.15407/pmach2020.04.063
- Nocera, D. G. (2012). The Artificial Leaf. Accounts of Chemical Research, 45 (5), 767–776. doi: https://doi.org/10.1021/ar2003013
- Felgenhauer, M., Hamacher, T. (2015). State-of-the-art of commercial electrolyzers and on-site hydrogen generation for logistic vehicles in South Carolina. International Journal of Hydrogen Energy, 40 (5), 2084–2090. doi: https://doi.org/10.1016/j.ijhydene.2014.12.043
- Krivtcova, V. I., Levterov, A. A., Grushko, A. I. (2006). Analiz pozharovzryvobezopasnosti sistem khraneniia i podachi vodoroda na osnove reaktcii samorasprostraniaiushchegosia vysokotemperaturnogo sinteza intermetallidov. Problemi nadzvichainikh situatcіi, 3, 145–151.
- Vasylkovskyi, O. M., Leshchenko, S. M., Vasylkovska, K. V., Petrenko, D. I. (2016). Pidruchnyk doslidnyka. Kirovohrad, 204.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Olha Lysenko, Valerii Ikonnikov

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







