Adsorption removal of copper(II) from water by zero valent iron loaded dendritic mesoporous silica
DOI:
https://doi.org/10.15587/2706-5448.2024.314231Keywords:
mesoporous silica, modification, adsorption, water treatment, heavy metals, DMSNAbstract
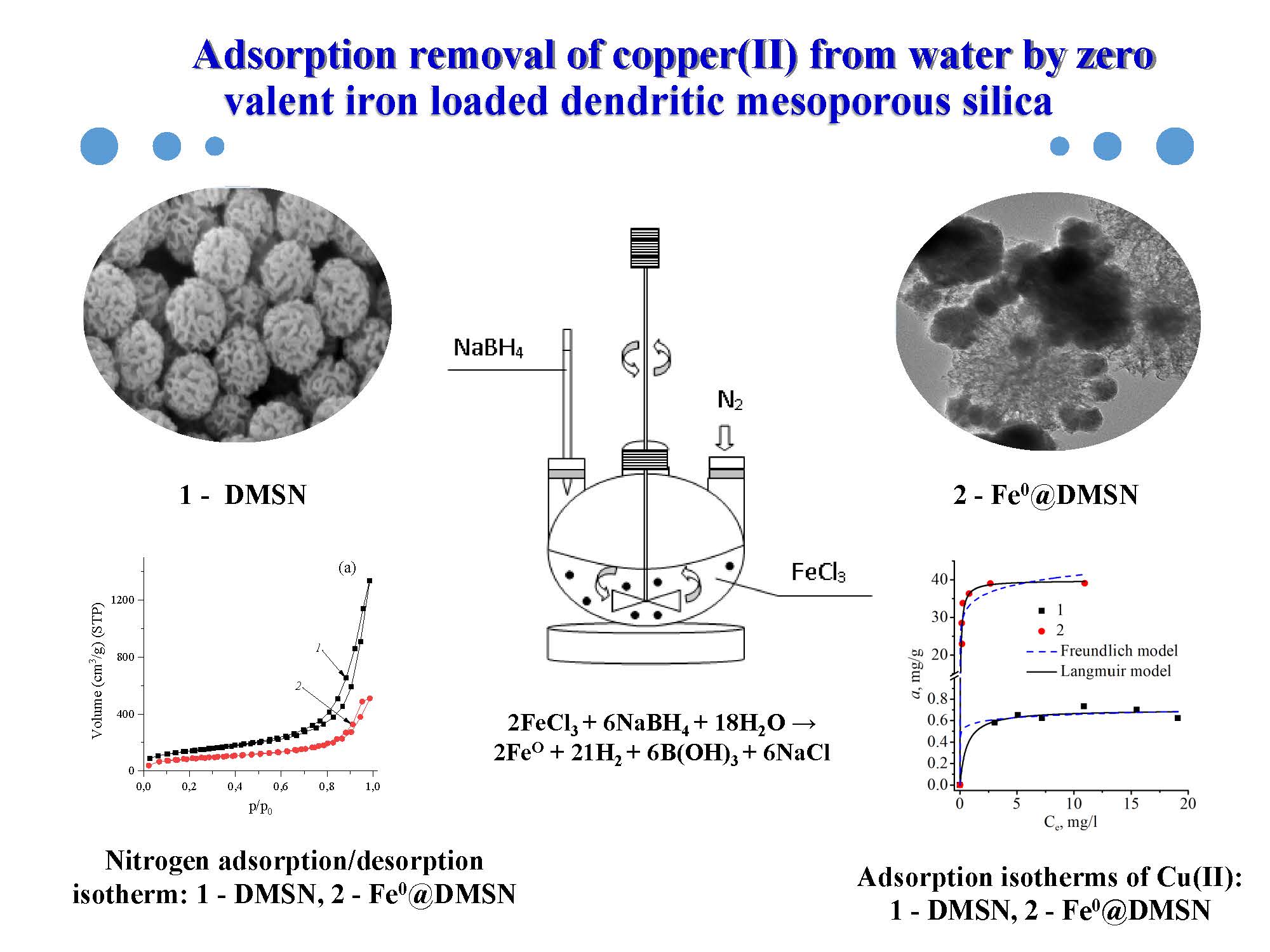
The object of research is synthesized dendritic mesoporous nanoscale silica (DMSN) modified with zero-valent iron (Fe0@DMSN). This material exhibits a high adsorption capacity for heavy metal ions, in particular copper, whose increased content in the aquatic environment poses a threat to living organisms. In this regard, the main physicochemical features of the removal of copper cations from the aqueous medium using the obtained sample were investigated.
The morphology of the obtained dendritic silicas was studied by electron microscopy and the presence of a layer of zero-valent iron was confirmed by X-ray diffraction analysis and infrared spectroscopy. The parameters of the porous structure of the synthesized materials were determined. It was found that after modification of mesoporous silica with particles of zero-valent iron, the value of its specific surface area decreased from 504 m2/g to 312 m2/g. This may be due to the formation of a Fe0 layer not only on their surface but also in the channels of the inorganic matrix, which has a unique dendritic structure characteristic of this type of particles. At the same time, the number of active centers increases due to the enrichment of the silica surface with functional modifier groups that show a high affinity for metal cations.
The adsorption capacity of Fe0@DMSN towards Cu2+ ions has been studied and it has been shown that the maximum adsorption value is 39.8 mg/g, which is significantly higher than that of the initial synthesized DMSN sample (0.7 mg/g).
The experimental data obtained indicate that the obtained sorption material based on dendritic mesoporous silica nanoparticles with a layer of reactive zero-valent iron can be used for the purification of water contaminated with metal ions. In addition, the magnetic properties of such materials, known and proven by various scientists, will make it easy to separate the solid phase in the processes of sorption water purification using magnetic separation.
References
- Zamora-Ledezma, C., Negrete-Bolagay, D., Figueroa, F., Zamora-Ledezma, E., Ni, M., Alexis, F., Guerrero, V. H. (2021). Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environmental Technology & Innovation, 22, 101504. https://doi.org/10.1016/j.eti.2021.101504
- Jiang, J., Wang, X., Ren, H., Cao, G., Xie, G., Xing, D., Liu, B. (2020). Investigation and fate of microplastics in wastewater and sludge filter cake from a wastewater treatment plant in China. Science of The Total Environment, 746, 141378. https://doi.org/10.1016/j.scitotenv.2020.141378
- Gao, J., Zhang, L., Liu, S., Liu, X. (2022). Enhanced adsorption of copper ions from aqueous solution by two-step DTPA-modified magnetic cellulose hydrogel beads. International Journal of Biological Macromolecules, 211, 689–699. https://doi.org/10.1016/j.ijbiomac.2022.05.073
- Krstić, V., Urošević, T., Pešovski, B. (2018). A review on adsorbents for treatment of water and wastewaters containing copper ions. Chemical Engineering Science, 192, 273–287. https://doi.org/10.1016/j.ces.2018.07.022
- Ibrahim, Y., Naddeo, V., Banat, F., Hasan, S. W. (2020). Preparation of novel polyvinylidene fluoride (PVDF)-Tin(IV) oxide (SnO2) ion exchange mixed matrix membranes for the removal of heavy metals from aqueous solutions. Separation and Purification Technology, 250, 117250. https://doi.org/10.1016/j.seppur.2020.117250
- Shi, X., Duan, Z., Jing Wang, Zhou, W., Jiang, M., Li, T., Ma, H., Zhu, X. (2023). Simultaneous removal of multiple heavy metals using single chamber microbial electrolysis cells with biocathode in the micro-aerobic environment. Chemosphere, 318, 137982. https://doi.org/10.1016/j.chemosphere.2023.137982
- Peydayesh, M., Mohammadi, T., Nikouzad, S. K. (2020). A positively charged composite loose nanofiltration membrane for water purification from heavy metals. Journal of Membrane Science, 611, 118205. https://doi.org/10.1016/j.memsci.2020.118205
- Xiao, Y., Tan, S., Wang, D., Wu, J., Jia, T., Liu, Q. et al. (2020). CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Applied Surface Science, 530, 147116. https://doi.org/10.1016/j.apsusc.2020.147116
- Feng, X., Long, R., Wang, L., Liu, C., Bai, Z., Liu, X. (2022). A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Separation and Purification Technology, 284, 120099. https://doi.org/10.1016/j.seppur.2021.120099
- Gupta, M., Gupta, H., Kharat, D. S. (2018). Adsorption of Cu(II) by low cost adsorbents and the cost analysis. Environmental Technology & Innovation, 10, 91–101. https://doi.org/10.1016/j.eti.2018.02.003
- Rubab, R., Ali, S., Rehman, A. U., Khan, S. A., Khan, A. M. (2021). Templated synthesis of NiO/SiO2 nanocomposite for dye removal applications: Adsorption kinetics and thermodynamic properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 615, 126253. https://doi.org/10.1016/j.colsurfa.2021.126253
- Zhang, S., Gao, H., Li, J., Huang, Y., Alsaedi, A., Hayat, T. et al. (2017). Rice husks as a sustainable silica source for hierarchical flower-like metal silicate architectures assembled into ultrathin nanosheets for adsorption and catalysis. Journal of Hazardous Materials, 321, 92–102. https://doi.org/10.1016/j.jhazmat.2016.09.004
- Vojoudi, H., Badiei, A., Bahar, S., Mohammadi Ziarani, G., Faridbod, F., Ganjali, M. R. (2017). A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. Journal of Magnetism and Magnetic Materials, 441, 193–203. https://doi.org/10.1016/j.jmmm.2017.05.065
- Shao, P., Liang, D., Yang, L., Shi, H., Xiong, Z., Ding, L. et al. (2020). Evaluating the adsorptivity of organo-functionalized silica nanoparticles towards heavy metals: Quantitative comparison and mechanistic insight. Journal of Hazardous Materials, 387, 121676. https://doi.org/10.1016/j.jhazmat.2019.121676
- Li, S., Li, S., Wen, N., Wei, D., Zhang, Y. (2021). Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica. Separation and Purification Technology, 265, 118341. https://doi.org/10.1016/j.seppur.2021.118341
- Yang, Y., Bernardi, S., Song, H., Zhang, J., Yu, M., Reid, J. C. et al. (2016). Anion Assisted Synthesis of Large Pore Hollow Dendritic Mesoporous Organosilica Nanoparticles: Understanding the Composition Gradient. Chemistry of Materials, 28 (3), 704–707. https://doi.org/10.1021/acs.chemmater.5b03963
- Zhang, K., Xu, L.-L., Jiang, J.-G., Calin, N., Lam, K.-F., Zhang, S.-J. et al. (2013). Facile Large-Scale Synthesis of Monodisperse Mesoporous Silica Nanospheres with Tunable Pore Structure. Journal of the American Chemical Society, 135 (7), 2427–2430. https://doi.org/10.1021/ja3116873
- Gao, F., Lei, C., Liu, Y., Song, H., Kong, Y., Wan, J., Yu, C. (2021). Rational Design of Dendritic Mesoporous Silica Nanoparticles’ Surface Chemistry for Quantum Dot Enrichment and an Ultrasensitive Lateral Flow Immunoassay. ACS Applied Materials & Interfaces, 13 (18), 21507–21515. https://doi.org/10.1021/acsami.1c02149
- Shi, L., Lin, Y.-M., Zhang, X., Chen, Z. (2011). Synthesis, characterization and kinetics of bentonite supported nZVI for the removal of Cr(VI) from aqueous solution. Chemical Engineering Journal, 171 (2), 612–617. https://doi.org/10.1016/j.cej.2011.04.038
- Dong, K., Wu, S., Chang, B., Sun, T. (2023). Zero-valent iron supported by dendritic mesoporous silica nanoparticles to purify dye wastewater. Journal of Environmental Chemical Engineering, 11 (5), 110434. https://doi.org/10.1016/j.jece.2023.110434
- Li, H., Si, R., Wang, W., Huang, Y., Xiang, M., Wang, C. et al. (2021). Sulfidated nanoscale zero-valent iron dispersed in dendritic mesoporous silica nanospheres for degrading tetrabromobisphenol A. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 621, 126586. https://doi.org/10.1016/j.colsurfa.2021.126586
- Yu, J., Bondarieva, A., Tobilko, V., Pavlenko, V. (2023). Adsorption removal of Cu(II) using Ni-modified silica gel. Water and Water Purification Technologies. Scientific and Technical News, 37 (3), 3–12.
- Choi, W. S., Lee, H.-J. (2022). Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes. Polymers, 14 (11), 2183. https://doi.org/10.3390/polym14112183
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Junjie Yu, Viktoriia Tobilko

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







