Increasing the sorption capacity of the native form of clinoptylolite for Mn2+ ions to obtain sorbents modified with manganese oxides
DOI:
https://doi.org/10.15587/2706-5448.2025.323870Keywords:
sorption capacity, manganese(II) ions, manganese oxides, oxidative catalysis, water purification, iron ions, hydrogen sulphideAbstract
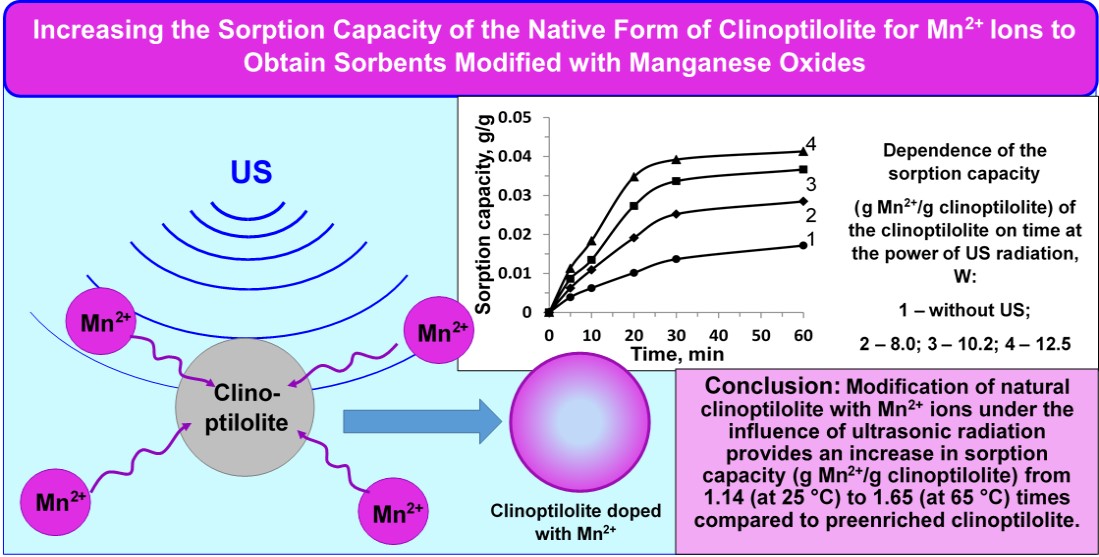
The object of the research was the process of sorption of Mn2+ ions by natural clinoptilolite (native form) under the influence of ultrasonic (US) radiation for the subsequent production of sorbents modified with manganese oxides, which have additional catalytic and oxidative capacity. Such sorbents with additional functions will be widely used in water purification processes from iron and manganese ions, hydrogen sulphide and a number of organic compounds, as well as highly dispersed and colloidal particles. This will allow combining the processes of purification of dispersed particles and soluble compounds of Fe2+, Mn2+, sulphides. The research was carried out with a clinoptilolite fraction of 1.0–1.5 mm, which is used in water purification processes. It was found that the native form of clinoptilolite has a lower sorption capacity for Mn2+ compared to clinoptilolite previously enriched by washing out impurities. The process of modifying clinoptilolite under the influence of ultrasound made it possible to significantly increase the sorption capacity of the zeolite for Mn2+ ions, compared not only to the native form of clinoptilolite, but also to the previously enriched one. Thus, at ultrasound powers of 8.0; 10.2 and 12.5 W, the sorption capacity of the native form of clinoptilolite increased by 1.66; 2.14 and 2.41 times, compared to the control experiment (without ultrasound). Compared to the enriched clinoptilolite, an increase in sorption capacity is also observed, although somewhat smaller: at powers of 8.0; 10.2 and 12.5 W it increased by 1.14; 1.47 and 1.65 times. It was found that the increase in temperature has little effect on the value of the sorption capacity of clinoptilolite. The value of the temperature coefficient g close to 1.1 indicates the course of the process in the diffusion region. EDX analysis has shown that the sorption of Mn2+ ions occurs mainly by the mechanism of selective ion exchange. The sorption capacity of clinoptilolite modified under adiabatic conditions is lower than under isothermal conditions. However, this method of modification has prospects at a higher mass ratio between the modification solution and zeolite. The results obtained have prospects for use in obtaining sorbents based on natural clinoptilolite with additional catalytic properties.
References
- Lei, L., Yao, Z., Zhou, J., Zheng, W., Wei, B., Zu, J., Yan, K. (2021). Hydrangea-like Ni/NiO/C composites derived from metal-organic frameworks with superior microwave absorption. Carbon, 173, 69–79. https://doi.org/10.1016/j.carbon.2020.10.093
- Hao, L., Meng, X., Wang, C., Wu, Q., Wang, Z. (2019). Preparation of nickel-doped nanoporous carbon microspheres from metal-organic framework as a recyclable magnetic adsorbent for phthalate esters. Journal of Chromatography A, 1605, 460364. https://doi.org/10.1016/j.chroma.2019.460364
- Liu, X., Wang, C., Wu, Q., Wang, Z. (2015). Metal-organic framework-templated synthesis of magnetic nanoporous carbon as an efficient absorbent for enrichment of phenylurea herbicides. Analytica Chimica Acta, 870, 67–74. https://doi.org/10.1016/j.aca.2015.02.036
- Li, D., He, M., Chen, B., Hu, B. (2019). Metal organic frameworks-derived magnetic nanoporous carbon for preconcentration of organophosphorus pesticides from fruit samples followed by gas chromatography-flame photometric detection. Journal of Chromatography A, 1583, 19–27. https://doi.org/10.1016/j.chroma.2018.11.012
- Wei, X., Wang, Y., Chen, J., Xu, F., Liu, Z., He, X. et al. (2020). Adsorption of pharmaceuticals and personal care products by deep eutectic solvents-regulated magnetic metal-organic framework adsorbents: Performance and mechanism. Chemical Engineering Journal, 392, 124808. https://doi.org/10.1016/j.cej.2020.124808
- Mashkuri, A., Saljooqi, A., Tohidiyan, Z. (2017). Nano clay Ni/NiO nanocomposite new sorbent for separation and preconcentration dibenzothiophene from crude prior to UV–vis spectrophotometery determination. Analytical Chemistry Research, 12, 47–51. https://doi.org/10.1016/j.ancr.2017.02.002
- Mastinu, A., Kumar, A., Maccarinelli, G., Bonini, S. A., Premoli, M., Aria, F. et al. (2019). Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules, 24 (8), 1517. https://doi.org/10.3390/molecules24081517
- Brambilla, D., Mancuso, C., Scuderi, M. R., Bosco, P., Cantarella, G., Lempereur, L., Di Benedetto, G., Pezzino, S., Bernardini, R. (2008). The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: a point of view for an assessment of the risk/benefit profile. Nutrition Journal, 7 (1), 29–38. https://doi.org/10.1186/1475-2891-7-29
- Reháková, M., Čuvanová, S., Dzivák, M., Rimár, J., Gaval’ová, Z. (2004). Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Current Opinion in Solid State and Materials Science, 8 (6), 397–404. https://doi.org/10.1016/j.cossms.2005.04.004
- Mkilima, T., Devrishov, D., Assel, K., Ubaidulayeva, N., Tleukulov, A., Khassenova, A. et al. (2022). Natural Zeolite for The Purification of Saline Groundwater and Irrigation Potential Analysis. Molecules, 27 (22), 7729. https://doi.org/10.3390/molecules27227729
- Margeta, K., Zabukovec, N., Siljeg, M., Farkas, A. (2013). Natural Zeolites in Water Treatment – How Effective is Their Use. Water Treatment. https://doi.org/10.5772/50738
- Pandová, I., Rimár, M., Panda, A., Valíček, J., Kušnerová, M., Harničárová, M. (2020). A Study of Using Natural Sorbent to Reduce Iron Cations from Aqueous Solutions. International Journal of Environmental Research and Public Health, 17 (10), 3686. https://doi.org/10.3390/ijerph17103686
- Znak, Z., Zin, O., Mashtaler, A., Korniy, S., Sukhatskiy, Yu., Gogate, P. R. et al. (2021). Improved modification of clinoptilolite with silver using ultrasonic radiation. Ultrasonics Sonochemistry, 73, 105496. https://doi.org/10.1016/j.ultsonch.2021.105496
- Colella, C. (2005). Natural zeolites. Zeolites and Ordered Mesoporous Materials: Progress and Prospects, 13–40. https://doi.org/10.1016/s0167-2991(05)80004-7
- Ivanenko, O. I., Krysenko, D. A., Krysenko, T. V., Tobilko, V. Yu. (2020). Use of natural zeolite of sokyrnytsа deposit for obtaining oxide-manganese catalyst for carbon monoxide oxidation. Visnyk KhNTU, 3 (74), 26–37. https://doi.org/10.35546/kntu2078-4481.2020.3.3
- Znak, Z., Kochubei, V. (2023). Influence of Natural Clinoptilolite Modification with Ions and Zero-Valent Silver on Its Sorption Capacity. Chemistry & Chemical Technology, 17 (3), 646–654. https://doi.org/10.23939/chcht17.03.646
- Pyrih, M. A., Znak, Z. O. (2024). Study of sorption of Мn2+ ions by natural clinoptilolite. Chemistry, Technology and Application of Substances, 7 (2), 40–46. https://doi.org/10.23939/ctas2024.02.040
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Zenovii Znak, Marta Pyrih

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







