Determination of the effectiveness of fungicide protection systems as a reserve for sustainable sunflower production in south of Ukraine
DOI:
https://doi.org/10.15587/2706-5448.2025.323971Keywords:
white mold, black stem, stem canker, downy mildew, disease development, fungicidal protection, sunflower, productivityAbstract
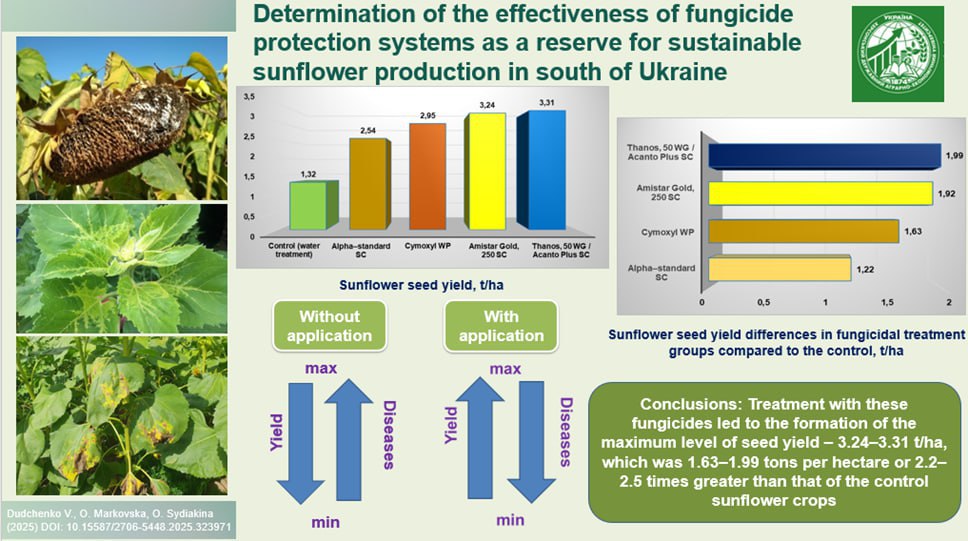
Sunflower is a strategic crop in the agricultural sector of Ukraine, but its yield and seed quality are significantly reduced due to damage from phytopathogens. One of the main methods for their control is the use of fungicides. A study conducted in the southern regions of Ukraine established the impact of fungicide application schemes based on active substances from the classes of benzimidazoles, strobilurins, and triazoles on the spread and severity of dominant diseases and sunflower yield. The main diseases of the crop included white mold, downy mildew, black stem, and stem canker. In the absence of fungicidal protection, disease development at BBCH growth stage 91 was significant, reaching 17.5 % (white mold), 28.9 % (downy mildew), 15.3 %, and 14.5 % for black stem and stem canker, respectively. The best biological efficacy for controlling Sclerotinia sclerotiorum and Phoma macdonaldii at BBCH growth stage 16 was shown by Amistar Gold, 250 SC (1.0 l/ha) – 78.0 % and 84.3 %, respectively. Against Diaporthe helianthi and Plasmopara halstedii, the highest efficacy at the same growth stage was demonstrated by Thanos, 50 WG – 86.6 % and 92.7 %, respectively. The use of Amistar Gold, 250 SC and Acanto Plus SC at BBCH growth stage 51 showed high biological efficacy against black stem and stem canker, ranging from 88.8 % to 90.3 %. Against downy mildew, the efficacy of Acanto Plus SC was higher by 4.5 % compared to Amistar Gold, 250 SC, reaching 88.9 %. For white mold, the efficacy of these products ranged from 80.0 % to 85.7 %, with Acanto Plus SC being more effective. Alpha-standard SC did not affect the development of downy mildew and showed low efficacy against white mold – 17.1 %. For black stem and stem canker, it had average efficacy rates of 74.5 % and 77.2 %, respectively. The use of fungicide application schemes: Amistar Gold, 250 SC (BBCH 16; 51) and Thanos, 50 WG (BBCH 16) along with Acanto Plus SC (BBCH 51) provided maximum yields of 3.24 and 3.31 t/ha, exceeding the control by 1.63 and 1.99 t/ha, respectively. The recommended protection schemes using Amistar Gold, 250 SC (1.0 l/ha), Thanos, 50 WG (0.6 kg/ha), and Acanto Plus SC (1.0 l/ha) can be implemented in farms in southern Ukraine and adapted to other sunflower growing zones.
References
- Pinkovskyi, H., Tanchyk, S. (2020). Productivity and economic efficiency of growing sunflower depending on the sowing time and plant density in the Right-Bank Steppe of Ukraine. Agrobìologìâ, 2 (161), 115–123. https://doi.org/10.33245/2310-9270-2020-161-2-115-123
- Chuiko, D. V., Ponomarova, M. S., Brahin, O. M. (2021). Economic efficiency of growing lines, hybrids and varieties of sunflower dependent from the plant growth regulator. Visnyk KhNAU. Seriia: Ekonomichni nauky, 1 (2), 197–208.
- Shyshkin, V., Onyshchenko, O. (2020). Present state and prospects of agricultural development of Ukraine. Management and Entrepreneurship: Trends of Development, 3 (13), 72–86. https://doi.org/10.26661/2522-1566/2020-3/13-06
- Trojanová, Z., Sedlářová, M., Gulya, T. J., Lebeda, A. (2016). Methodology of virulence screening and race characterization of Plasmopara halstedii, and resistance evaluation in sunflower – a review. Plant Pathology, 66 (2), 171–185. Portico. https://doi.org/10.1111/ppa.12593
- Sackston, W. E.; Spencer, D. E. (Ed.) (1981). Downy mildew of sunflower. The Downy Mildews. London: Academic Press, 545–575.
- Young, P. A., Morris, H. E. (1927). Plasmopara downy mildew of cultivated sunflowers. American Journal of Botany, 14 (9), 551–552. https://doi.org/10.1002/j.1537-2197.1927.tb04866.x
- Gulya, T. J, Miler, J. F., Viranyi, F., Sackston, W. E. (1991). Proposed internationally standardized method for race identification of Plasmopara halstedii. Helia, 14, 11–20.
- Mathew, F., Harveson, R., Block, C., Gulya, T., Ryley, M., Thompson, S., Markell, S. (2020). Sclerotinia Diseases of Sunflower. Plant Health Instructor, 23, 23–27. https://doi.org/10.1094/phi-i-2020-1201-01
- Harveson, R. M., Markell, S. G., Block, C. C., Gulya, T. J. (2016). Introduction. Compendium of Sunflower Diseases and Pests, 1–13. https://doi.org/10.1094/9780890545096.001
- Rashid, K. Y., Block, C. C., Gulya, T. J. (2016). Sclerotinia Head Rot and Midstalk Rot. Compendium of sunflower diseases and pests. St. Paul: American Phytopathological Society Press, 51–55.
- Pikovskyi, M. Y., Kyryk, M. M. (2021). Bioekolohichni osoblyvosti fitopatohennykh hrybiv Sclerotinia sclerotiorum (Lib.) de Bary i Botryotinia fuckeliana (de Bary) Whetzel. Kyiv: FOP Yamchynskyi O.V., 278.
- Holley, R. C., Nelson, B. D. (1986). Effect of Plant Population and Inoculum Density on Incidence of Sclerotinia Wilt of Sunflower. Phytopathology, 76 (1), 71–74. https://doi.org/10.1094/phyto-76-71
- Quiroz, F. J., Edwards Molina, J. P., Dosio, G. A. A. (2014). Black stem by Phoma macdonaldii affected ecophysiological components that determine grain yield in sunflower (Helianthus annuus L.). Field Crops Research, 160, 31–40. https://doi.org/10.1016/j.fcr.2014.02.011
- Dedić, B. (2016). Phoma macdonaldii Boerema, prouzrokovač crne pegavosti stabla suncokreta – varijabilnost populacije i iznalaženje izvora otpornosti. [Doctoral dissertation; University of Novi Sad].
- Thompson, S. M., Tan, Y. P., McTaggart, A. R., Shivas, R. G. (2016). Subcommittee on Plant Health Diagnostics. National Diagnostic Protocol for Diaporthe helianthi – NDP40 V1. Eds. Subcommittee on Plant Health Diagnostics.
- Mathew, F., Block, C., Harveson, R., Gulya, T., Ryley, M., Thompson, S., Markell, S. (2019). Diaporthe helianthi (stem canker of sunflower). CABI Compendium. CABI Publishing. https://doi.org/10.1079/cabicompendium.18733
- Mathew, F., Harveson, R., Gulya, T., Thompson, S., Block, C., Markell, S. (2018). Phomopsis Stem Canker of Sunflower. Plant Health Instructor, 18 (1). https://doi.org/10.1094/phi-i-2018-1103-01
- Kareem, F. H., Matloob, A. A. (2020). Efficiency of some of bio–formulas against fungi caused sunflower root rot disease. International Journal of Agricultural and Statistical Sciences, 16 (1), 1485–1493. https://connectjournals.com/03899.2020.16.1485
- Moin, S., Ali, S. A., Hasan, K. A., Tariq, A., Sultana, V., Ara, J., Ehteshamul-Haque, S. (2020). Managing the root rot disease of sunflower with endophytic fluorescent Pseudomonas associated with healthy plants. Crop Protection, 130, 105066. https://doi.org/10.1016/j.cropro.2019.105066
- Pospelov, S. V., Pospielova, G. D., Nechiporenko, N. I., Mishchenko, O. V., Cherniak О. О., Skliar, S. S., Ivanichko, O. V. (2021). Analysis of sunflower areas’ phyto-pathogenic condition during vegetation period under different agro-climatic conditions. Scientific Progress & Innovations, 4, 133–141. https://doi.org/10.31210/visnyk2021.04.17
- Andriichuk, T., Skoreiko, A., Kuvshynov, O. (2021). Evaluation of phytosanitary condition of sunflower crops in the Western Forest-Steppe of Ukraine. Interdepartmental Thematic Scientific Collection of Plant Protection and Quarantine, 67, 73–84. https://doi.org/10.36495/1606-9773.2021.67.73-84
- Kgatle, M. G., Flett, B., Truter, M., Aveling, T. A. S. (2020). Control of Alternaria leaf blight caused by Alternaria alternata on sunflower using fungicides and Bacillus amyloliquefaciens. Crop Protection, 132, 105146. https://doi.org/10.1016/j.cropro.2020.105146
- Melnychuk, F. S., Marchenko, O. A., Vasyliev, A. A. (2020). The influence of irrigation on the phytopathogenic complex on sunflower under the conditions of the Forest-Steppe of Ukraine. Taurian Scientific Herald, 2 (116), 32–40. https://doi.org/10.32851/2226-0099.2020.116.2.5
- Trybel, S. O., Siharova, D. D., Sekun, M. P., Ivashchenko, O. O. et al. (2001). Metody vyprobuvannia ta zastosuvannia pestytsydiv. Kyiv: Svit, 448.
- Stankevych, S. V., Zabrodina, I. V., Vasylieva, Yu. V., Turenko, V. P., Kuleshov, A. V., Bilyk, M. O. (2020). Monitorynh shkidnykiv i khvorob silskohospodarskykh kultur. Kharkiv: FOP Brovin O. V., 624. Available at: https://repo.btu.kharkov.ua//handle/123456789/24118
- Ushkarenko, V. O., Nikishenko, V. L., Holoborodko, S. P., Kokovikhin, S. V. (2008). Dyspersiino-koreliatsiinyi analiz u silskomu hospodarstvi ta roslynnytstvi. Kherson: Ailant, 272.
- Bilai, V. I. (1982). Metody eksperymentalnoi mikolohii. Kyiv: Naukova dumka, 552.
- Abbott, W. S. (1925). A Method of Computing the Effectiveness of an Insecticide. Journal of Economic Entomology, 18 (2), 265–267. https://doi.org/10.1093/jee/18.2.265a
- Kyryk, M. M., Pikovskyi, M. Y. (2011). Fitopatolohichnyi monitorynh. Kyiv: TsP KOMPRYNT, 248.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Volodymyr Dudchenko, Olena Markovska, Olena Sydiakina

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







