Research into arsenic (III) effective catalytic oxidation in an aqueous solution on a new active manganese dioxide in a flow column
DOI:
https://doi.org/10.15587/2706-5448.2024.298969Keywords:
groundwater, removal of arsenic from water, arsenic (III) oxidation, arsenic (III) oxidation catalysts, arsenic sorption, manganese dioxideAbstract
Groundwater in many places on Earth contains arsenic compounds. Arsenic (III) compounds must be oxidised to purify water containing arsenic effectively. The subject of this study is oxidation of arsenic (III) compounds in an aqueous solution.
Today’s most common industrial arsenic oxidation method using aggressive oxidising agents such as chlorine or ozone has a number of serious disadvantages. The most problematic of these include extremely high risks to human health and the environment, the cost and overall complexity of the process. Catalytic oxidation of arsenic (III) compounds using atmospheric oxygen is an alternative free from the above disadvantages, yet, to date, no information about effective catalysts for this process has been presented in the literature.
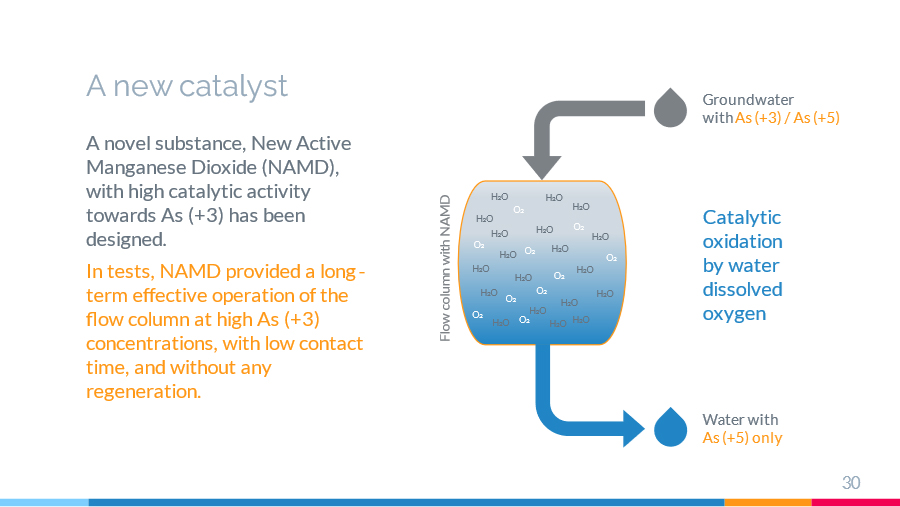
The arsenic (III) catalytic oxidation process is studied in an aqueous solution on a new active manganese dioxide (NAMD) synthesised by the author. A comparative experimental analysis is performed with other known modifications of manganese dioxide. It is shown that the new active manganese dioxide (NAMD) has high catalytic activity towards arsenic (III), this being confirmed experimentally in both a limited volume and a flow column mode. Some theoretical aspects of the mechanism for catalytic oxidation of arsenic (III) with oxygen on active manganese dioxide in an aqueous solution are also discussed on the basis of the research results.
Experimental work is required at pilot plants in the field for successful industrial implementation of the technology for catalytic oxidation of arsenic (III) compounds on NAMD. Further laboratory research is necessary for developing a detailed theoretical basis for catalytic oxidation of arsenic in aqueous solutions.
The results of this research are of interest to industrial companies specialising in removing arsenic compounds from water, to scientists and researchers studying catalytic oxidation of arsenic (III), as well as heterogeneous catalytic oxidation with oxygen in general.
References
- Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., Divya, B. V. (2021). Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geoscience Frontiers, 12 (3), 101079. doi: https://doi.org/10.1016/j.gsf.2020.08.015
- Rajakovic, L., Rajakovic-Ognjanovic, V. (2018). Arsenic in Water: Determination and Removal. Arsenic – Analytical and Toxicological Studies. doi: https://doi.org/10.5772/intechopen.75531
- Hering, J. G., Katsoyiannis, I. A., Theoduloz, G. A., Berg, M., Hug, S. J. (2017). Arsenic Removal from Drinking Water: Experiences with Technologies and Constraints in Practice. Journal of Environmental Engineering, 143 (5). doi: https://doi.org/10.1061/(asce)ee.1943-7870.0001225
- Ghurye, G., Clifford, D. (2004). As(III) oxidation using chemical and solid‐phase oxidants. Journal AWWA, 96 (1), 84–96. doi: https://doi.org/10.1002/j.1551-8833.2004.tb10536.x
- Zhang, W., Singh, P., Issa, T. B. (2011). Arsenic(III) Remediation from Contaminated Water by Oxidation and Fe/Al Co-Precipitation. Journal of Water Resource and Protection, 3 (9), 655–660. doi: https://doi.org/10.4236/jwarp.2011.39075
- Ghurye, V. (2001). Laboratory study on the oxidation of Arsenic III to Arsenic Clifford. University of Houston.
- Pokhrel, R., Goetz, M. K., Shaner, S. E., Wu, X., Stahl, S. S. (2015). The “Best Catalyst” for Water Oxidation Depends on the Oxidation Method Employed: A Case Study of Manganese Oxides. Journal of the American Chemical Society, 137 (26), 8384–8387. doi: https://doi.org/10.1021/jacs.5b05093
- Bajpai, S., Chaudhuri, M. (1999). Removal of Arsenic from Ground Water by Manganese Dioxide–Coated Sand. Journal of Environmental Engineering, 125 (8), 782–784. doi: https://doi.org/10.1061/(asce)0733-9372(1999)125:8(782)
- Villalobos, M., Escobar-Quiroz, I. N., Salazar-Camacho, C. (2014). The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As(V) and oxidation of As(III). Geochimica et Cosmochimica Acta, 125, 564–581. doi: https://doi.org/10.1016/j.gca.2013.10.029
- Ajith, N., Dalvi, A. A., Swain, K. K., Devi, P. S. R., Kalekar, B. B., Verma, R., Reddy, A. V. R. (2013). Sorption of As(III) and As(V) on chemically synthesized manganese dioxide. Journal of Environmental Science and Health, Part A, 48 (4), 422–428. doi: https://doi.org/10.1080/10934529.2013.728919
- Dalvi, A. A., Ajith, N., Swain, K. K., Verma, R. (2015). Sorption of arsenic on manganese dioxide synthesized by solid state reaction. Journal of Environmental Science and Health, Part A, 50 (8), 866–873. doi: https://doi.org/10.1080/10934529.2015.1019808
- Shih, Y.-J., Huang, R.-L., Huang, Y.-H. (2015). Adsorptive removal of arsenic using a novel akhtenskite coated waste goethite. Journal of Cleaner Production, 87, 897–905. doi: https://doi.org/10.1016/j.jclepro.2014.10.065
- Oscarson, D. W., Huang, P. M., Defosse, C., Herbillon, A. (1981). Oxidative power of Mn(IV) and Fe(III) oxides with respect to As(III) in terrestrial and aquatic environments. Nature, 291 (5810), 50–51. doi: https://doi.org/10.1038/291050a0
- Oscarson, D. W., Huang, P. M., Liaw, W. K., Hammer, U. T. (1983). Kinetics of Oxidation of Arsenite by Various Manganese Dioxides. Soil Science Society of America Journal, 47 (4), 644–648. doi: https://doi.org/10.2136/sssaj1983.03615995004700040007x
- Scott, M. J., Morgan, J. J. (1996). Reactions at Oxide Surfaces. 2. Oxidation of Se(IV) by Synthetic Birnessite. Environmental Science & Technology, 30 (6), 1990–1996. doi: https://doi.org/10.1021/es950741d
- Manning, B. A., Fendorf, S. E., Bostick, B., Suarez, D. L. (2002). Arsenic(III) Oxidation and Arsenic(V) Adsorption Reactions on Synthetic Birnessite. Environmental Science & Technology, 36 (5), 976–981. doi: https://doi.org/10.1021/es0110170
- Kariakin, Iu. V. (1947). Chistye khimicheskie reaktivy. Moscow, 574.
- Murray, J. W. (1974). The surface chemistry of hydrous manganese dioxide. Journal of Colloid and Interface Science, 46 (3), 357–371. doi: https://doi.org/10.1016/0021-9797(74)90045-9
- McKenzie, R. (1970). The reaction of cobalt with manganese dioxide minerals. Soil Research, 8 (1), 97–106. doi: https://doi.org/10.1071/sr9700097
- Driehaus, W., Seith, R., Jekel, M. (1995). Oxidation of arsenate(III) with manganese oxides in water treatment. Water Research, 29 (1), 297–305. doi: https://doi.org/10.1016/0043-1354(94)e0089-o
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Denis Abower

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







