Дослідження ефективного каталітичного окиснення миш'яку (III) у водному розчині на новому активному діоксиді марганцю в проточній колоні
DOI:
https://doi.org/10.15587/2706-5448.2024.298969Ключові слова:
ґрунтові води, очищення води від миш'яку, окислення миш'яку (III), каталізатори окислення миш'яку (III), сорбція миш'яку, діоксид марганцюАнотація
У багатьох місцях Землі ґрунтові води містять сполуки миш'яку. Для ефективного очищення води, що містить миш'як, з'єднання миш'яку (III) необхідно окислювати. Об'єктом цього дослідження є окислення сполук миш'яку (III) у водному розчині.
Промислова технологія окислення миш'яку, що застосовується сьогодні в більшості випадків, агресивними окислювачами, такими як хлор або озон, має ряд серйозних недоліків. До найбільш проблематичних з них відносяться вкрай високі ризики для здоров'я людей та навколишнього середовища, собівартість та загальна трудомісткість процесу. Каталітичне окиснення сполук миш'яку (III) киснем повітря є вільною від перерахованих вище недоліків альтернативою, проте дотепер відомостей про ефективність каталізаторів даного процесу в літературі не представлено.
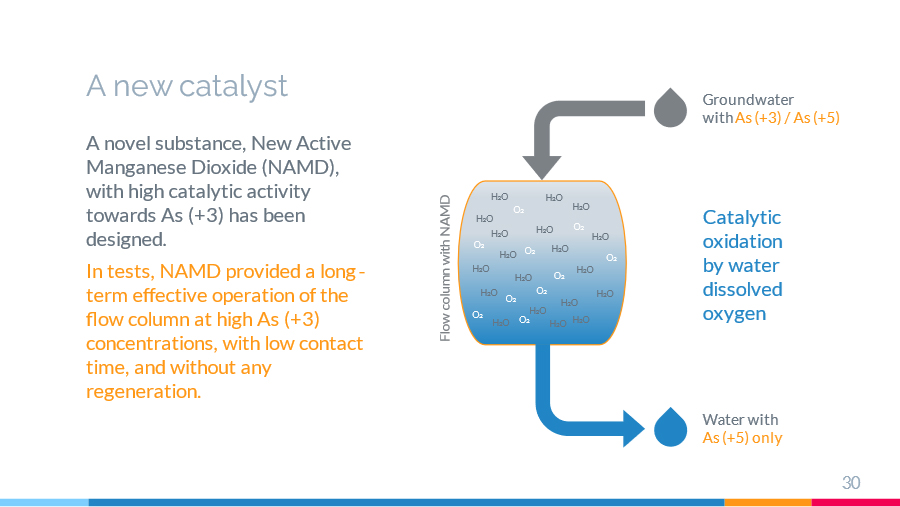
У цій роботі досліджується процес каталітичного окислення миш'яку (III) у водному розчині на синтезованому автором новому активному діоксиді марганцю (НАДМ). Проводиться порівняльний експериментальний аналіз з іншими відомими модифікаціями діоксиду марганцю. Показано, що новий активний діоксид марганцю (НАДМ) має високу каталітичну активність по відношенню до миш'яку (III), що підтверджено експериментально як в обмеженому обсязі, так і в режимі проточної колони. За результатами дослідження також обговорюються деякі теоретичні аспекти механізму каталітичного окиснення миш'яку (III) киснем активного діоксиду марганцю у водному розчині.
Для успішного промислового впровадження технології каталітичного окиснення сполук миш'яку (III) на НАДМ необхідно проведення дослідних робіт на пілотних установках у польових умовах. Для розробки детального теоретичного обґрунтування механізму каталітичного окиснення миш'яку у водних розчинах необхідні подальші лабораторні дослідження.
Результати даної роботи становлять інтерес як для промислових компаній, що спеціалізуються на очищенні води від сполук миш'яку, так і для вчених та дослідників, які вивчають каталітичне окислення миш'яку (III), а також гетерогенне каталітичне окислення киснем в цілому.
Посилання
- Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., Divya, B. V. (2021). Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geoscience Frontiers, 12 (3), 101079. doi: https://doi.org/10.1016/j.gsf.2020.08.015
- Rajakovic, L., Rajakovic-Ognjanovic, V. (2018). Arsenic in Water: Determination and Removal. Arsenic – Analytical and Toxicological Studies. doi: https://doi.org/10.5772/intechopen.75531
- Hering, J. G., Katsoyiannis, I. A., Theoduloz, G. A., Berg, M., Hug, S. J. (2017). Arsenic Removal from Drinking Water: Experiences with Technologies and Constraints in Practice. Journal of Environmental Engineering, 143 (5). doi: https://doi.org/10.1061/(asce)ee.1943-7870.0001225
- Ghurye, G., Clifford, D. (2004). As(III) oxidation using chemical and solid‐phase oxidants. Journal AWWA, 96 (1), 84–96. doi: https://doi.org/10.1002/j.1551-8833.2004.tb10536.x
- Zhang, W., Singh, P., Issa, T. B. (2011). Arsenic(III) Remediation from Contaminated Water by Oxidation and Fe/Al Co-Precipitation. Journal of Water Resource and Protection, 3 (9), 655–660. doi: https://doi.org/10.4236/jwarp.2011.39075
- Ghurye, V. (2001). Laboratory study on the oxidation of Arsenic III to Arsenic Clifford. University of Houston.
- Pokhrel, R., Goetz, M. K., Shaner, S. E., Wu, X., Stahl, S. S. (2015). The “Best Catalyst” for Water Oxidation Depends on the Oxidation Method Employed: A Case Study of Manganese Oxides. Journal of the American Chemical Society, 137 (26), 8384–8387. doi: https://doi.org/10.1021/jacs.5b05093
- Bajpai, S., Chaudhuri, M. (1999). Removal of Arsenic from Ground Water by Manganese Dioxide–Coated Sand. Journal of Environmental Engineering, 125 (8), 782–784. doi: https://doi.org/10.1061/(asce)0733-9372(1999)125:8(782)
- Villalobos, M., Escobar-Quiroz, I. N., Salazar-Camacho, C. (2014). The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As(V) and oxidation of As(III). Geochimica et Cosmochimica Acta, 125, 564–581. doi: https://doi.org/10.1016/j.gca.2013.10.029
- Ajith, N., Dalvi, A. A., Swain, K. K., Devi, P. S. R., Kalekar, B. B., Verma, R., Reddy, A. V. R. (2013). Sorption of As(III) and As(V) on chemically synthesized manganese dioxide. Journal of Environmental Science and Health, Part A, 48 (4), 422–428. doi: https://doi.org/10.1080/10934529.2013.728919
- Dalvi, A. A., Ajith, N., Swain, K. K., Verma, R. (2015). Sorption of arsenic on manganese dioxide synthesized by solid state reaction. Journal of Environmental Science and Health, Part A, 50 (8), 866–873. doi: https://doi.org/10.1080/10934529.2015.1019808
- Shih, Y.-J., Huang, R.-L., Huang, Y.-H. (2015). Adsorptive removal of arsenic using a novel akhtenskite coated waste goethite. Journal of Cleaner Production, 87, 897–905. doi: https://doi.org/10.1016/j.jclepro.2014.10.065
- Oscarson, D. W., Huang, P. M., Defosse, C., Herbillon, A. (1981). Oxidative power of Mn(IV) and Fe(III) oxides with respect to As(III) in terrestrial and aquatic environments. Nature, 291 (5810), 50–51. doi: https://doi.org/10.1038/291050a0
- Oscarson, D. W., Huang, P. M., Liaw, W. K., Hammer, U. T. (1983). Kinetics of Oxidation of Arsenite by Various Manganese Dioxides. Soil Science Society of America Journal, 47 (4), 644–648. doi: https://doi.org/10.2136/sssaj1983.03615995004700040007x
- Scott, M. J., Morgan, J. J. (1996). Reactions at Oxide Surfaces. 2. Oxidation of Se(IV) by Synthetic Birnessite. Environmental Science & Technology, 30 (6), 1990–1996. doi: https://doi.org/10.1021/es950741d
- Manning, B. A., Fendorf, S. E., Bostick, B., Suarez, D. L. (2002). Arsenic(III) Oxidation and Arsenic(V) Adsorption Reactions on Synthetic Birnessite. Environmental Science & Technology, 36 (5), 976–981. doi: https://doi.org/10.1021/es0110170
- Kariakin, Iu. V. (1947). Chistye khimicheskie reaktivy. Moscow, 574.
- Murray, J. W. (1974). The surface chemistry of hydrous manganese dioxide. Journal of Colloid and Interface Science, 46 (3), 357–371. doi: https://doi.org/10.1016/0021-9797(74)90045-9
- McKenzie, R. (1970). The reaction of cobalt with manganese dioxide minerals. Soil Research, 8 (1), 97–106. doi: https://doi.org/10.1071/sr9700097
- Driehaus, W., Seith, R., Jekel, M. (1995). Oxidation of arsenate(III) with manganese oxides in water treatment. Water Research, 29 (1), 297–305. doi: https://doi.org/10.1016/0043-1354(94)e0089-o
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2024 Denis Abower

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.








