Study of technological aspects of manufacture of polymer composite material by centrifugal fiber forming method
DOI:
https://doi.org/10.15587/2706-5448.2024.310805Keywords:
hesperidin, solid dispersion system, polymer, centrifugal formation of fibers, polymer composite materialAbstract
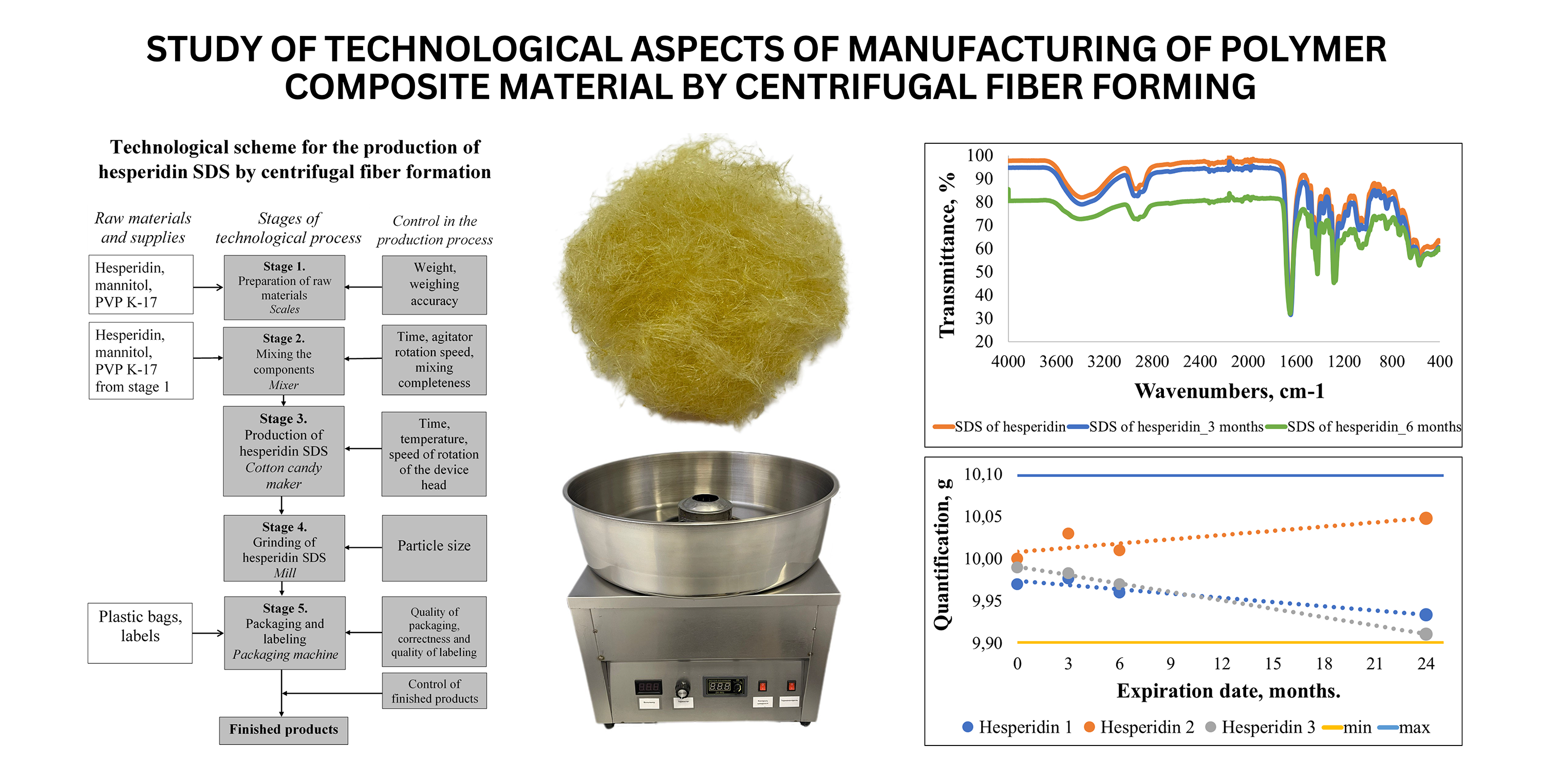
The object of the study is the technological aspects of manufacturing the hesperidin polymer composite material by the method of centrifugal fiber formation. This method is considered the basis of a relatively new and cost-effective way of producing solid dispersion systems. Using the centrifugal molding method, it is possible to obtain highly soluble forms of active pharmaceutical ingredients in the form of fibers of various sizes using a wide range of polymeric materials with high speed and low cost due to simple equipment. Due to the innovative design of the centrifugal fiber formation method, it was chosen for the development of solid dispersion systems of the bioflavonoid hesperidin, which has a wide range of different pharmacological properties, but low bioavailability.
Solid dispersed systems of hesperidin by the method of centrifugal fiber formation were produced on the basis of a pharmaceutically acceptable polymeric carrier of polyvinylpyrrolidone and mannitol. For the obtained solid dispersed systems, such basic pharmaco-technological characteristics as loss in mass during drying, bulk volume, bulk volume after shrinkage, bulk density, bulk density after shrinkage, compressibility index, Gaussner coefficient were determined.
Comprehensive tests of the stability of the studied samples of the solid dispersion system of hesperidin were carried out under the conditions of accelerated tests for 6 months. According to the obtained results, it was established that the developed polymer composite material is stable in the studied conditions, and its conditional shelf life is 2 years.
A technological scheme for the production of the hesperidin polymer composite material in the form of solid dispersed systems by the method of centrifugal fiber formation has been developed. In particular, the technological process is described step by step and the critical indicators of quality control of the obtained composite material are determined. The proposed technology can be implemented in modern chemical and pharmaceutical industries. This will contribute to the expansion of the market of highly effective socially oriented medicines.
Supporting Agency
- Volodymyr Bessarabov was supported by the Ministry of Education and Science of Ukraine [0122U000139].
References
- Li, J., Yu, F., Chen, Y., Oupický, D. (2015). Polymeric drugs: Advances in the development of pharmacologically active polymers. Journal of Controlled Release, 219, 369–382. https://doi.org/10.1016/j.jconrel.2015.09.043
- Adams, J. S., Sutar, Y., Dhoble, S., Maiti, C., Hanjankar, S. N., Das, R. et al. (2024). Pharmaceutical and biomedical polymers: Basics, modifications, and applications. Polymers for Pharmaceutical and Biomedical Applications, 1–86. https://doi.org/10.1016/b978-0-323-95496-9.00001-6
- Nair, A. R., Lakshman, Y. D., Anand, V. S. K., Sree, K. S. N., Bhat, K., Dengale, S. J. (2020). Overview of Extensively Employed Polymeric Carriers in Solid Dispersion Technology. AAPS PharmSciTech, 21 (8). https://doi.org/10.1208/s12249-020-01849-z
- Malkawi, R., Malkawi, W. I., Al-Mahmoud, Y., Tawalbeh, J. (2022). Current Trends on Solid Dispersions: Past, Present, and Future. Advances in Pharmacological and Pharmaceutical Sciences, 2022, 1–17. https://doi.org/10.1155/2022/5916013
- Tran, P., Pyo, Y.-C., Kim, D.-H., Lee, S.-E., Kim, J.-K., Park, J.-S. (2019). Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics, 11 (3), 132. https://doi.org/10.3390/pharmaceutics11030132
- Satish, S., Priya, R. (2022). A mini review on centrifugal spinning technique for production of nanofibers and its applications in drug delivery. Journal of Medical Pharmaceutical and Allied Sciences, 11 (1), 4349–4352. https://doi.org/10.55522/jmpas.v11i1.2176
- Zhang, X., Lu, Y. (2014). Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polymer Reviews, 54 (4), 677–701. https://doi.org/10.1080/15583724.2014.935858
- Farhaj, S., Conway, B. R., Ghori, M. U. (2023). Nanofibres in Drug Delivery Applications. Fibers, 11 (2), 21. https://doi.org/10.3390/fib11020021
- Marano, S., Barker, S. A., Raimi-Abraham, B. T., Missaghi, S., Rajabi-Siahboomi, A., Craig, D. Q. M. (2016). Development of micro-fibrous solid dispersions of poorly water-soluble drugs in sucrose using temperature-controlled centrifugal spinning. European Journal of Pharmaceutics and Biopharmaceutics, 103, 84–94. https://doi.org/10.1016/j.ejpb.2016.03.021
- Nasir, S., Hussain, A., Abbas, N., Bukhari, N. I., Hussain, F., Arshad, M. S. (2021). Improved bioavailability of oxcarbazepine, a BCS class II drug by centrifugal melt spinning: In-vitro and in-vivo implications. International Journal of Pharmaceutics, 604, 120775. https://doi.org/10.1016/j.ijpharm.2021.120775
- Hussain, A., Hussain, F., Arshad, M. S., Abbas, N., Nasir, S., Mudassir, J. et al. (2021). Ibuprofen-loaded centrifugally spun microfibers for quick relief of inflammation in rats. Drug Development and Industrial Pharmacy, 47 (11), 1786–1793. https://doi.org/10.1080/03639045.2022.2059500
- Priya, R., Satish, S. (2023). Centrifugal Melt Spun Microfi brous Solid Dispersion of Diclofenac Sodium with Enhanced Solubility. International journal of pharmaceutical quality assurance, 14 (1), 165–170. https://doi.org/10.25258/ijpqa.14.1.28
- Alfassam, H. A., Booq, R. Y., Almousained, M. M., Alajmi, A. M., Elfaky, M. A., Shaik, R. A. et al. (2024). Fabrication and evaluation of centrifugal spun Miconazole-loaded sugar-based fibers. Journal of Drug Delivery Science and Technology, 98, 105872. https://doi.org/10.1016/j.jddst.2024.105872
- Choi, S.-S., Lee, S.-H., Lee, K.-A. (2022). A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants, 11 (8), 1618. https://doi.org/10.3390/antiox11081618
- Donia, T., Dabbour, N. M., Loutfy, S. A.; Xiao, J. (Ed.) (2023). Hesperidin: Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. Handbook of Dietary Flavonoids. Cham: Springer, 1–55. https://doi.org/10.1007/978-3-030-94753-8_28-1
- European Pharmacopoeia 9.0 Volume 1. (2018). Strasbourg: Council of Europe.
- Heorhiievskyi, V., Liapunov, M., Bezuhla, O. et al. (2004). Nastanova z yakosti. Likarski zasoby. Vyprobuvannia stabilnosti. Nastanova 42-3.3.2004. Ministerstvo okhorony zdorovia Ukrainy. Kyiv: Morion, 60.
- Lisovyi, V., Bessarabov, V., Goy, A., Kostiuk, V. (2024). Spectrophotometric method for determining the assay of hesperidin in the composition of polymer composite material obtained by centrifugal fiber formation. Herald of Khmelnytskyi National University. Technical Sciences, 335 (3 (1)), 135–141. https://doi.org/10.31891/2307-5732-2024-335-3-19
- Kurakula, M., Rao, G. S. N. K. (2020). Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. Journal of Drug Delivery Science and Technology, 60, 102046. https://doi.org/10.1016/j.jddst.2020.102046
- Hureieva, S. M., Kondratova, Yu. A. (2016). Vyvchennia stabilnosti likarskoho zasobu Antral, tabletky, vkryti obolonkoiu. Farmatsevtychnyi zhurnal, 2, 70–76.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Vadym Lisovyi, Volodymyr Bessarabov

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







