Development of iron-containing adsorbents for fluoride ion removal
DOI:
https://doi.org/10.15587/2706-5448.2025.331608Keywords:
fluoride ion removal, iron, granular adsorbents, water treatment sludge, kinetic models, isothermsAbstract
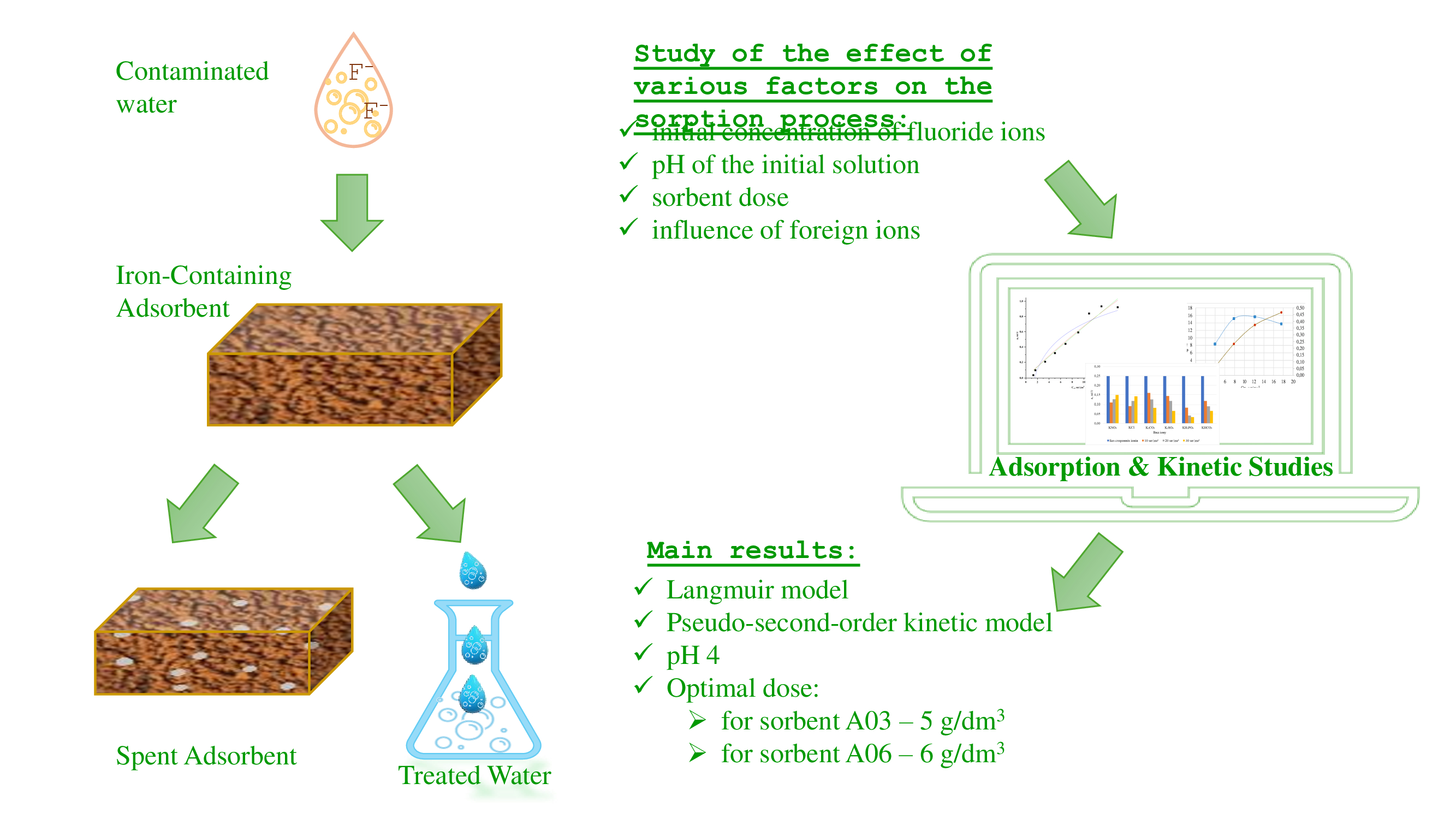
Ingestion of too much fluoride ions through drinking water can seriously harm human health. Adsorption is one of the most effective approaches that have been proposed for removing fluoride ions from the aquatic environment. Analysis of modern publications shows that the search for new effective sorbents obtained by resource-saving technologies is an urgent scientific and practical problem. It is proposed to use sediments from groundwater deironing stations as sorbents. These sludges are formed in significant quantities and create significant environmental problems. Therefore, the object of the study is samples of agglomerated iron-containing adsorbents.
Two samples of sorbents with different iron contents were studied. The influence of various parameters on the efficiency of fluoride ion adsorption was analyzed: contact time, initial fluoride concentration and adsorbent dose, pH value of the initial solution, and the presence of competing ions.
The experimental data fit well with the pseudo-second-order kinetic model (coefficient of determination R2 = 0.8581 for sample A03 and R2 = 0.9947 for A06). The best correlation of the experimental data with the Langmuir model is the coefficient of determination R2 = 0.965 for A03 and for A06 R2 = 0.970. It was found that the maximum efficiency was achieved at pH 4. With an increase in the initial fluoride concentration, the sorption capacity increases, and the removal efficiency first increases and then decreases.
For the sorbent A03, the optimal dose is 5 g/dm3, and for A06 – 6 g/dm3. The study of the influence of foreign ions on the sorption of fluoride ions on the sorbent showed that all the studied ions to some extent worsen the defluoridation efficiency.
The use of the proposed sorbent will allow solving the following environmental issues: replenishing the list of cheap Ukrainian sorbents for fluoride removal and utilization of sludge from iron removal stations.
Supporting Agency
- The research was conducted without financial support on initiative topics with State Registration Numbers: 0124U001966, 0124U002058.
References
- Zhao, M., Wang, Q., Krua, L. S. N., Yi, R., Zou, R., Li, X., Huang, P. (2023). Application Progress of New Adsorption Materials for Removing Fluorine from Water. Water, 15 (4), 646. https://doi.org/10.3390/w15040646
- Mariappan Santhi, V., Periasamy, D., Perumal, M., Sekar, P. M., Varatharajan, V., Aravind, D. et al. (2024). The Global Challenge of Fluoride Contamination: A Comprehensive Review of Removal Processes and Implications for Human Health and Ecosystems. Sustainability, 16 (24), 11056. https://doi.org/10.3390/su162411056
- Zhao, X., Li, Y., Carroll, K. C., Li, F., Qiu, L., Huo, Z. (2021). Mesoporous goethite for rapid and high-capacity fluoride removal from drinking water. Journal of Environmental Chemical Engineering, 9 (4), 105278. https://doi.org/10.1016/j.jece.2021.105278
- Chiavola, A., D’Amato, E., Di Marcantonio, C. (2022). Comparison of Adsorptive Removal of Fluoride from Water by Different Adsorbents under Laboratory and Real Conditions. Water, 14 (9), 1423. https://doi.org/10.3390/w14091423
- Bhatnagar, A., Kumar, E., Sillanpää, M. (2011). Fluoride removal from water by adsorption – A review. Chemical Engineering Journal, 171 (3), 811–840. https://doi.org/10.1016/j.cej.2011.05.028
- Ahmad, S., Singh, R., Arfin, T., Neeti, K. (2022). Fluoride contamination, consequences and removal techniques in water: a review. Environmental Science: Advances, 1 (5), 620–661. https://doi.org/10.1039/d1va00039j
- El Messaoudi, N., Franco, D. S. P., Gubernat, S., Georgin, J., Şenol, Z. M., Ciğeroğlu, Z. et al. (2024). Advances and future perspectives of water defluoridation by adsorption technology: A review. Environmental Research, 252, 118857. https://doi.org/10.1016/j.envres.2024.118857
- Gebrewold, B. D., Werkneh, A. A., Kijjanapanich, P., Rene, E. R., Lens, P. N. L., Annachhatre, A. P. (2024). Low cost materials for fluoride removal from groundwater. Journal of Environmental Management, 370, 122937. https://doi.org/10.1016/j.jenvman.2024.122937
- Kurylenko, V., Tolstopalova, N., Sanginova, O., Obushenko, T. (2023). Review of fluorine removal methods from aqueous solutions. Proceedings of the NTUU “Igor Sikorsky KPI”. Series: Chemical Engineering, Ecology and Resource Saving, 1, 52–69. https://doi.org/10.20535/2617-9741.1.2023.276447
- Kurylenko, V., Tolstopalova, N., Obushenko, T., Sanginova, O., Dontsova, T. (2023). Fluoride ions removal efficiency of natural/activated zeolite and bentonite sorbents. Water and water purification technologies. scientific and technical news, 36 (2), 27–39. https://doi.org/10.20535/2218-930022023300526
- Siaurusevičiūtė, I., Albrektienė, R. (2021). Removal of Fluorides from Aqueous Solutions Using Exhausted Coffee Grounds and Iron Sludge. Water, 13 (11), 1512. https://doi.org/10.3390/w13111512
- Mesbah, M., Hamedshahraki, S., Ahmadi, S., Sharifi, M., Igwegbe, C. A. (2020). Hydrothermal synthesis of LaFeO3 nanoparticles adsorbent: Characterization and application of error functions for adsorption of fluoride. MethodsX, 7, 100786. https://doi.org/10.1016/j.mex.2020.100786
- Obushenko, T., Tolstopalova, N., Sanginova, O., Kostenko, E., Bolielyi, O., Kurylenko, V. (2022). Study of adsorption of phosphate ions from aqueous solutions. Technology Audit and Production Reserves, 4 (3 (66)), 35–37. https://doi.org/10.15587/2706-5448.2022.264669
- Patel, D., Kulwant, M., Shirin, S., Varshney, R., Pandey, G., Yadav, A. K.; Yadav, A. K., Shirin, S., Singh, V. P. (Eds.) (2023). Fluoride Removal from Aqueous Solution Using Iron-Based Materials: Preparation, Characterization, and Applications. Advanced Treatment Technologies for Fluoride Removal in Water. Cham: Springer, 71–92. https://doi.org/10.1007/978-3-031-38845-3_4
- Revellame, E. D., Fortela, D. L., Sharp, W., Hernandez, R., Zappi, M. E. (2020). Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Cleaner Engineering and Technology, 1, 100032. https://doi.org/10.1016/j.clet.2020.100032
- Musah, M., Azeh, Y., Mathew, J., Umar, M., Abdulhamid, Z., Muhammad, A. (2022). Adsorption Kinetics and Isotherm Models: A Review. Caliphate Journal of Science and Technology, 4 (1), 20–26. https://doi.org/10.4314/cajost.v4i1.3
- Chen, X. (2015). Modeling of Experimental Adsorption Isotherm Data. Information, 6 (1), 14–22. https://doi.org/10.3390/info6010014
- Prathna, T. C., Sharma, S. K., Kennedy, M. (2017). Development of iron oxide nanoparticle adsorbents for arsenic and fluoride removal. Desalination and Water Treatment, 67, 187–195. https://doi.org/10.5004/dwt.2017.20464
- Babaeivelni, K., Khodadoust, A. P. (2013). Adsorption of fluoride onto crystalline titanium dioxide: Effect of pH, ionic strength, and co-existing ions. Journal of Colloid and Interface Science, 394, 419–427. https://doi.org/10.1016/j.jcis.2012.11.063
- Nur, T., Loganathan, P., Nguyen, T. C., Vigneswaran, S., Singh, G., Kandasamy, J. (2014). Batch and column adsorption and desorption of fluoride using hydrous ferric oxide: Solution chemistry and modeling. Chemical Engineering Journal, 247, 93–102. https://doi.org/10.1016/j.cej.2014.03.009
- de Carvalho Costa, L. R., Jurado-Davila, I. V., Oliveira, J. T. D., Nunes, K. G. P., Estumano, D. C., de Oliveira, R. A. et al. (2024). Exploring Key Parameters in Adsorption for Effective Fluoride Removal: A Comprehensive Review and Engineering Implications. Applied Sciences, 14 (5), 2161. https://doi.org/10.3390/app14052161
- Adamu, D. B., Zereffa, E., Segne, T. A., Razali, M. H., Lemu, B. R. (2023). Synthesis of iron-substituted hydroxyapatite nanomaterials by co-precipitation method for defluoridation. Materials Research Express, 10 (4), 045006. https://doi.org/10.1088/2053-1591/acca65
- Azari, A., Kalantary, R. R., Ghanizadeh, G., Kakavandi, B., Farzadkia, M., Ahmadi, E. (2015). Iron–silver oxide nanoadsorbent synthesized by co-precipitation process for fluoride removal from aqueous solution and its adsorption mechanism. RSC Advances, 5 (106), 87377–87391. https://doi.org/10.1039/c5ra17595j
- Cai, H., Chen, G., Peng, C., Xu, L., Zhu, X., Zhang, Z. et al. (2015). Enhanced removal of fluoride by tea waste supported hydrous aluminium oxide nanoparticles: anionic polyacrylamide mediated aluminium assembly and adsorption mechanism. RSC Advances, 5 (37), 29266–29275. https://doi.org/10.1039/c5ra01560j
- Emamjomeh, M. M., Sivakumar, M., Varyani, A. S. (2011). Analysis and the understanding of fluoride removal mechanisms by an electrocoagulation/flotation (ECF) process. Desalination, 275 (1-3), 102–106. https://doi.org/10.1016/j.desal.2011.02.032
- Jeyaseelan, A., Viswanathan, N., Kumar, I. A., Naushad, Mu. (2023). Design of hydrotalcite and biopolymers entrapped tunable cerium organic cubic hybrid material for superior fluoride adsorption. Colloids and Surfaces B: Biointerfaces, 224, 113190. https://doi.org/10.1016/j.colsurfb.2023.113190
- Jin, Z., Jia, Y., Zhang, K.-S., Kong, L.-T., Sun, B., Shen, W. et al. (2016). Effective removal of fluoride by porous MgO nanoplates and its adsorption mechanism. Journal of Alloys and Compounds, 675, 292–300. https://doi.org/10.1016/j.jallcom.2016.03.118
- Hernández-Campos, M., Polo, A. M. S., Sánchez-Polo, M., Rivera-Utrilla, J., Berber-Mendoza, M. S., Andrade-Espinosa, G., López-Ramón, M. V. (2018). Lanthanum-doped silica xerogels for the removal of fluorides from waters. Journal of Environmental Management, 213, 549–554. https://doi.org/10.1016/j.jenvman.2018.02.016
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Evgeniy Kostenko, Arcady Shakhnovsky, Tetiana Obushenko, Olga Sanginova, Nataliia Tolstopalova

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







