Фізико-хімічні властивості залізо(II) сульфат гептагідрату як чинники для вибору режиму процесу сушіння в апараті киплячого шару
DOI:
https://doi.org/10.15587/2706-5448.2025.335323Ключові слова:
залізо(II) сульфат гептагідрат, гранулометричний склад, вільна та кристалізаційна волога, домішкиАнотація
Об’єктом дослідження є залізо(II) сульфат гептагідрат – основний твердий відхід сульфатного виробництва діоксиду титану, накопичення якого становить значну екологічну загрозу. Проблемним місцем його переробки є технологічна стадія дегідратації в киплячому шару до моногідратної форми, для якої важливим є вибір прийнятного гідродинамічного режиму та режиму сушіння.
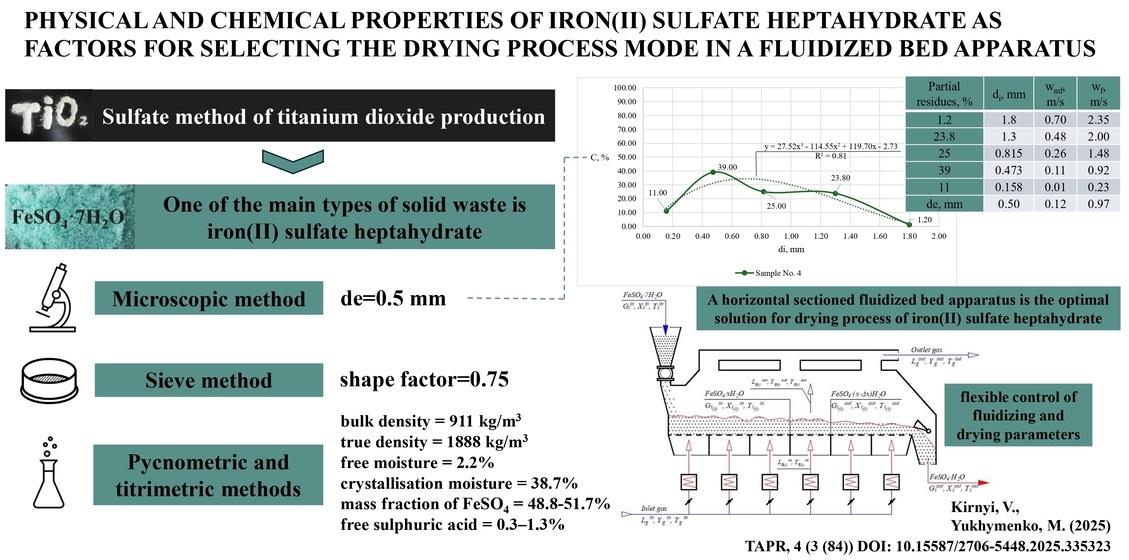
Експериментальні дослідження включали мікроскопічний, ситовий, пікнометричний та титрометричний методи аналізу. Встановлено середній еквівалентний діаметр частинок – 0,50 мм, коефіцієнт форми – 0,75. Насипна густина матеріалу складає 911 кг/м³, істинна густина – 1888 кг/м³. Вміст вільної вологи виявлено на рівні 2,2%, кристалізаційної вологи – 38,7%, що відповідає гептагідратній формі FeSO4 · 7H2O. Хімічний аналіз показав, що масова частка FeSO4 у зразках коливається від 48,8% до 51,7%, а вміст Fe2+ складає 18%. Вільна сірчана кислота присутня в кількості 0,3–1,3%.
Гранулометричний аналіз виявив значну полідисперсність матеріалу, зокрема наявність агломератів та дрібних фракцій зразків № 1–3 закритого складу зберігання залізо(II) сульфат гептагідрату. Для зразка № 4, який мав характеристики найбільш рівномірного за розподілом частинок, розраховано критичну швидкість початку зважування найбільшої за розміром частинок фракції (0,7 м/с) та швидкість витання (0,97 м/с) для еквівалентного діаметру частинок матеріалу. Встановлено, що частинки діаметром менш як 0,207 мм будуть виноситись із киплячого шару, що потребує додаткових заходів для зменшення втрат матеріалу. Коефіцієнт тепловіддачі для частинок проміжних фракцій (0,315–1,6 мм) становить 77,79–349,17 Вт/(м² · К), що забезпечує ефективний теплообмін у процесі сушіння.
На основі отриманих даних обґрунтовано вибір горизонтального секціонованого апарату киплячого шару. Запропонована конструкція передбачає поділ процесу на незалежні зони з індивідуальним контролем та регулюванням параметрів сушильного агента (температури та витрати). Завдяки цьому забезпечується можливість отримання стабільного гідродинамічного режиму для полідисперсних матеріалів та зниження впливу перемішування на рушійну силу процесу.
Отримані результати дозволяють прогнозувати поведінку матеріалу в апараті киплячого шару, розраховувати режими зважування та сушіння.
Посилання
- Titanium dioxide market size – industry report on share, growth trends & forecasts analysis (2025–2030). Mordor Intelligence. Available at: https://www.mordorintelligence.com/industry-reports/titanium-dioxide-market
- Titanium Dioxide Market size to increase by USD 6899.93 million between 2022 to 2027, market Segmentation by Application and Geography – Technavio (2024). PR Newswire. Available at: https://www.prnewswire.com/news-releases/titanium-dioxide-market-size-to-increase-by-usd-6899-93-million-between-2022-to-2027--market-segmentation-by-application-and-geography--technavio-302089174.html
- Das, R., Ambardekar, V., Pratim Bandyopadhyay, P.; Ali, H. M. (Ed.) (2022). Titanium Dioxide and Its Applications in Mechanical, Electrical, Optical, and Biomedical Fields. Titanium Dioxide – Advances and Applications. IntechOpen. https://doi.org/10.5772/intechopen.98805
- Khaustov, V. (2023). Prospects for the development of titanium dioxide production in Ukraine. Scientific Bulletin of International Association of Scientists. Series: Economy, Management, Security, Technologies, 2 (4). https://doi.org/10.56197/2786-5827/2023-2-4-2
- Barsukova, H. V., Savchenko-Pererva, M. Y. (2021). Development of technology for obtaining a mixed coagulant from the main waste of titanium production. Journal of Chemistry and Technologies, 28 (3), 289–297. https://doi.org/10.15421/082031
- Gao, Y., Yue, T., Sun, W., He, D., Lu, C., Fu, X. (2021). Acid recovering and iron recycling from pickling waste acid by extraction and spray pyrolysis techniques. Journal of Cleaner Production, 312, 127747. https://doi.org/10.1016/j.jclepro.2021.127747
- Gázquez, M. J., Contreras, M., Pérez-Moreno, S. M., Guerrero, J. L., Casas-Ruiz, M., Bolívar, J. P. (2021). A Review of the Commercial Uses of Sulphate Minerals from the Titanium Dioxide Pigment Industry: The Case of Huelva (Spain). Minerals, 11 (6), 575. https://doi.org/10.3390/min11060575
- Mončeková, M., Novotný, R., Koplík, J., Kalina, L., Bílek, V., Šoukal, F. (2016). Hexavalent Chromium Reduction by Ferrous Sulphate Heptahydrate Addition into the Portland Clinker. Procedia Engineering, 151, 73–79. https://doi.org/10.1016/j.proeng.2016.07.382
- Green, D. W., Southard, M. Z. (Eds.) (2019). Perry’s chemical engineers’ handbook. McGraw-Hill Education. Available at: https://www.accessengineeringlibrary.com/content/book/9780071834087
- Shaikh, L., Pandit, A., Ranade, V. (2013). Crystallisation of ferrous sulphate heptahydrate: Experiments and modelling. The Canadian Journal of Chemical Engineering, 91 (1), 47–53. https://doi.org/10.1002/cjce.20695
- Gázquez, M. J., Bolívar, J. P., García-Tenorio, R., Vaca, F. (2009). Physicochemical characterization of raw materials and co-products from the titanium dioxide industry. Journal of Hazardous Materials, 166 (2-3), 1429–1440. https://doi.org/10.1016/j.jhazmat.2008.12.067
- Wang, T., Debelak, K. A., Roth, J. A. (2007). Dehydration of iron (II) sulfate heptahydrate. Thermochimica Acta, 462 (1-2), 89–93. https://doi.org/10.1016/j.tca.2007.07.001
- ISO 13322-1:2014 Particle size analysis – Image analysis methods – Part 1: Static image analysis methods (2014). International Organization for Standardization. Available at: https://cdn.standards.iteh.ai/samples/51257/e5956ce29c644e71ad37738bd690a3cf/ISO-13322-1-2014.pdf.
- ISO 9276-1:1998 Representation of results of particle size analysis – Part 1: Graphical representation (1998). International Organization for Standardization. Available at: https://cdn.standards.iteh.ai/samples/25860/7bdb509b70814f5ba4526488f14b7ba5/ISO-9276-1-1998.pdf
- Ulusoy, U. (2023). A Review of Particle Shape Effects on Material Properties for Various Engineering Applications: From Macro to Nanoscale. Minerals, 13 (1), 91. https://doi.org/10.3390/min13010091
- Atamaniuk, V. M., Humnytskyi, Ya. M. (2013). Naukovi osnovy filtratsiinoho sushinnia dyspersnykh materialiv. Lviv: Vydavnytstvo Lvivskoi politekhniky, 276.
- ISO 3923-1:2018 Metallic powders – Determination of apparent density – Part 1: Funnel method (2018). International Organization for Standardization. Available at: https://cdn.standards.iteh.ai/samples/69219/73e768c2dd64481eb5d96ff1c0e0a6a2/ISO-3923-1-2018.pdf
- ISO 567:2021 Coke – Determination of bulk density in a small container (2021). International Organization for Standardization. Available at: https://cdn.standards.iteh.ai/samples/78697/97576b6a78994ee0b1deca87b9a59dea/ISO-567-2021.pdf
- DSTU 2463-94 Kuporos zaliznyi tekhnichnyi. Tekhnichni umovy (HOST 6981-94) (1994). Derzhavnyi Standart Ukrainy. Available at: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=52579
- Srinivasan, T. G., Vasudeva Rao, P. R. (2014). Free acidity measurement – A review. Talanta, 118, 162–171. https://doi.org/10.1016/j.talanta.2013.10.017
- Davidson, J. F., Clift, R., Harrison, D. (Eds.) (1985). Fluidization. Academic Press, 733.
- Tsiura, N., Kindzera, D., Huzova, I., Atamanyuk, V. (2021). Study of the kinetics of drying iron (II) sulfate heptahydrate by filtration method. ScienceRise, 1, 11–21. https://doi.org/10.21303/2313-8416.2021.001583
- Yukhymenko, M., Artyukhov, A., Ostroha, R., Artyukhova, N., Krmela, J., Bocko, J. (2021). Multistage Shelf Devices with Fluidized Bed for Heat-Mass Transfer Processes: Experimental Studies and Practical Implementation. Applied Sciences, 11 (3), 1159. https://doi.org/10.3390/app11031159
- Bachmann, P., Bück, A., Tsotsas, E. (2017). Experimental investigation and correlation of the Bodenstein number in horizontal fluidized beds with internal baffles. Powder Technology, 308, 378–387. https://doi.org/10.1016/j.powtec.2016.11.025
- Yukhymenko, M., Ostroha, R., Lytvynenko, A., Mikhajlovskiy, Y., Bocko, J. (2020). Cooling Process Intensification for Granular Mineral Fertilizers in a Multistage Fluidized Bed Device. In: Ivanov, V., Pavlenko, I., Liaposhchenko, O., Machado, J., Edl, M. (eds) Advances in Design, Simulation and Manufacturing III. DSMIE 2020. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-50491-5_24
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2025 Viacheslav Kirnyi, Mykola Yukhymenko

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.








