Choline monitoring to assess inflammatory hepatitis
DOI:
https://doi.org/10.15587/2519-8025.2024.325058Keywords:
choline biosensor, choline oxidase, esterase activity, hepatitis, inflammation monitoringAbstract
The aim of the study is to create a rapid and reliable biosensor for determining choline in biological media.
Materials and methods. Choline oxidase (EC 1.1.3.17, 17 U/mg) from Arthrobacter globiformis was purchased from Sigma. Hydrogen peroxide (30 %, v/v aqueous solution), choline chloride, and bovine serum albumin (V fraction) (BSA) were obtained from Sigma. Glutaraldehyde 25 % was obtained from Merck KGaA. Semipermeable terylene membrane, 12 µM thickness, 0.4 µM diameter of pore were obtained from the Institute Joint Institute of Nuclear Research. The body fluid samples from mice were obtained from the National University of Pharmacy, Ukraine.
The layer consisting of 5 µL of a solution comprising ChOx, BSA, and glutaraldehyde was formed on the inner surface of the ring-fixed semipermeable terylene film (working area Ø 2.4 mm) by creating a membrane. Subsequently, it was maintained at 4 °C for a period of 12 hours. The enzymatic membrane was mechanically affixed to the Pt electrode's surface, forming a biosensor. Chronoamperometric measurements were conducted with a custom-made potentiostat (Vilnius University, Life Sciences Centre, Institute of Biochemistry), utilizing a conventional three-electrode electrochemical cell comprising a platinum auxiliary electrode, a saturated Ag/AgCl reference electrode, and the biosensor as the working electrode.
To simulate inflammation, we recreated the model of acute toxic tetrachloromethane hepatitis. Hepatitis is an acute or chronic inflammation of the liver, caused by various factors: intoxication with household substances, poisons, drugs, alcohol, autoimmune, and infectious processes. To simulate inflammation, we recreated the model of acute toxic tetrachloromethane hepatitis by the method of O. V. Stefanov. The study was conducted at the Biomedical Research Laboratory of the Educational and Research Institute of Applied Pharmacy of the National University of Pharmacy.
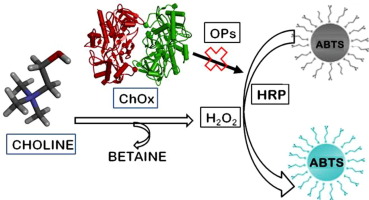
Results. A reagentless amperometric choline biosensor was developed and characterized using the enzyme choline oxidase from Arthrobacter globiformis (ChOx). The biosensor showed rapid response, appropriate stability, and sensitivity to choline when acting in model and in real biologic media. Since choline is a product of esterase-catalyzed reactions, the activity of esterases can be evaluated via choline release. This study revealed the increased concentrations of choline in the samples of the model of acute toxic tetrachloromethane hepatitis compared to control animals.
Conclusions. The ChOx based biosensor is a reliable tool for the monitoring of choline in biological media, such as blood serum. The activity of esterases can be evaluated via choline release. Consequently, measured esterase activity by choline-type biosensors could serve as biomarkers for the assessment of hepatitis-type inflammation dynamics.
This is also highly relevant for the study of the pharmacological action of drugs with the expected anti-inflammatory effect
References
- Alemany, M. (2024). The Metabolic Syndrome, a Human Disease. International Journal of Molecular Sciences, 25 (4), 2251. https://doi.org/10.3390/ijms25042251
- Khan, S., Mahgoub, S., Fallatah, N., Lalor, P. F., Newsome, P. N. (2023). Liver Disease and Cell Therapy: Advances Made and Remaining Challenges. Stem Cells, 41 (8), 739–761. https://doi.org/10.1093/stmcls/sxad029
- Hassan, G. S., Flores Molina, M., Shoukry, N. H. (2023). The multifaceted role of macrophages during acute liver injury. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1237042
- Michalik, M., Gładyś, A., Czekaj, P. (2020). Differentiation of Cells Isolated from Afterbirth Tissues into Hepatocyte-Like Cells and Their Potential Clinical Application in Liver Regeneration. Stem Cell Reviews and Reports, 17 (2), 581–603. https://doi.org/10.1007/s12015-020-10045-2
- Lam, L., Ilies, M. A. (2022). Evaluation of the Impact of Esterases and Lipases from the Circulatory System against Substrates of Different Lipophilicity. International Journal of Molecular Sciences, 23 (3), 1262. https://doi.org/10.3390/ijms23031262
- Rossaint, J., Margraf, A. (2020). Inflammation und perioperative Organdysfunktion. Der Anaesthesist, 70 (1), 83–92. https://doi.org/10.1007/s00101-020-00886-4
- Sreedhar, R., Watanabe, K., Arumugam, S. (2017). General Mechanisms of Immunity and Inflammation. Japanese Kampo Medicines for the Treatment of Common Diseases: Focus on Inflammation, 23–31. https://doi.org/10.1016/b978-0-12-809398-6.00003-2
- Rossaint, J., Margraf, A., Zarbock, A. (2019). Perioperative Inflammation. Der Anaesthesist, 68 (7), 421–427. https://doi.org/10.1007/s00101-019-0596-9
- Macovei, L. A., Birsan, M., Teodor, V. I., Cristofor, A. C., Ioanid, N., Rezus, E. (2017). On the Role of Chemical and Molecular Biology in Inflammation Research. Revista de Chimie, 68 (4), 786–788. https://doi.org/10.37358/rc.17.4.5553
- La Rosa, S. L., Lindstad, L. J., Westereng, B. (2023). Carbohydrate esterases involved in deacetylation of food components by the human gut microbiota. Essays in Biochemistry, 67 (3), 443–454. https://doi.org/10.1042/ebc20220161
- Ukegawa, T., Komatsu, T., Minoda, M., Matsumoto, T., Iwasaka, T., Mizuno, T. et al. (2023). Thioester‐Based Coupled Fluorogenic Assays in Microdevice for the Detection of Single‐Molecule Enzyme Activities of Esterases with Specified Substrate Recognition. Advanced Science, 11 (10). https://doi.org/10.1002/advs.202306559
- Petri, Y. D., Verresen, R., Gutierrez, C. S., Kojasoy, V., Zhang, E., Abularrage, N. S. et al. (2024). Mammalian Esterase Activity: Implications for Peptide Prodrugs. Biochemistry, 63 (20), 2580–2593. https://doi.org/10.1021/acs.biochem.4c00446
- Fu, Q., Wang, Y., Yan, C., Xiang, Y. K. (2024). Phosphodiesterase in heart and vessels: from physiology to diseases. Physiological Reviews, 104 (2), 765–834. https://doi.org/10.1152/physrev.00015.2023
- Kilanowska, A., Ziółkowska, A. (2020). Role of Phosphodiesterase in the Biology and Pathology of Diabetes. International Journal of Molecular Sciences, 21 (21), 8244. https://doi.org/10.3390/ijms21218244
- Sekaran, S., Vimalraj, S., Thangavelu, L. (2021). The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization. Biomolecules, 11 (11), 1564. https://doi.org/10.3390/biom11111564
- Haarhaus, M., Cianciolo, G., Barbuto, S., La Manna, G., Gasperoni, L., Tripepi, G. et al. (2022). Alkaline Phosphatase: An Old Friend as Treatment Target for Cardiovascular and Mineral Bone Disorders in Chronic Kidney Disease. Nutrients, 14 (10), 2124. https://doi.org/10.3390/nu14102124
- Borza, R., Salgado-Polo, F., Moolenaar, W. H., Perrakis, A. (2022). Structure and function of the ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP) family: Tidying up diversity. Journal of Biological Chemistry, 298 (2), 101526. https://doi.org/10.1016/j.jbc.2021.101526
- Jo, M., Knapp, M., Boggs, D. G., Brimberry, M., Donnan, P. H., Bridwell-Rabb, J. (2023). A structure-function analysis of chlorophyllase reveals a mechanism for activity regulation dependent on disulfide bonds. Journal of Biological Chemistry, 299 (3), 102958. https://doi.org/10.1016/j.jbc.2023.102958
- Ahmed, A. I., Abou-Taleb, K. A. A., Abd-Elhalim, B. T. (2023). Characterization and application of tannase and gallic acid produced by co-fungi of Aspergillus niger and Trichoderma viride utilizing agro-residues substrates. Scientific Reports, 13 (1). https://doi.org/10.1038/s41598-023-43955-5
- Hubbard, R. E., O’Mahony, M. S., Calver, B. L., Woodhouse, K. W. (2008). Plasma esterases and inflammation in ageing and frailty. European Journal of Clinical Pharmacology, 64 (9), 895–900. https://doi.org/10.1007/s00228-008-0499-1
- De Boer, D., Nguyen, N., Mao, J., Moore, J., Sorin, E. J. (2021). A Comprehensive Review of Cholinesterase Modeling and Simulation. Biomolecules, 11 (4), 580. https://doi.org/10.3390/biom11040580
- Efremenko, E., Maslova, O., Stepanov, N., Ismailov, A. (2021). Using Cholinesterases and Immobilized Luminescent Photobacteria for the Express-Analysis of Mycotoxins and Estimating the Efficiency of Their Enzymatic Hydrolysis. Toxins, 13 (1), 34. https://doi.org/10.3390/toxins13010034
- Břízová, A., Pitschmann, V. (2023). Simple Chemical and Cholinesterase Methods for the Detection of Nerve Agents Using Optical Evaluation. Biosensors, 13 (12), 995. https://doi.org/10.3390/bios13120995
- Garmavy, H. M. S., Mohammed, A. A., Rashid, H. M., Mohammad, F. K. (2023). A meta-analysis of normal human blood cholinesterase activities determined by a modified electrometric method. Journal of Medicine and Life, 16 (1), 22–34. https://doi.org/10.25122/jml-2022-0215
- Pradhan, B., Pandey, S., Niroula, A., Adhikari, N., Chapagain, N., Pradhan, S. (2023). Mean Cholinesterase Level among Organophosphorus Poisoning Patients Visiting the Emergency Department in a Tertiary Care Centre: A Descriptive Cross-sectional Study. Journal of Nepal Medical Association, 61 (257), 72–75. https://doi.org/10.31729/jnma.7983
- Danaei, G.-H., Amali, A., Karami, M., Khorrami, M.-B., Riahi-Zanjani, B., Sadeghi, M. (2022). The significance of thymoquinone administration on liver toxicity of diazinon and cholinesterase activity; a recommendation for prophylaxis among individuals at risk. BMC Complementary Medicine and Therapies, 22 (1). https://doi.org/10.1186/s12906-022-03806-8
- Bernhard, W., Raith, M., Shunova, A., Lorenz, S., Böckmann, K., Minarski, M. et al. (2022). Choline Kinetics in Neonatal Liver, Brain and Lung –Lessons from a Rodent Model for Neonatal Care. Nutrients, 14 (3), 720. https://doi.org/10.3390/nu14030720
- Arshad, U., Zenobi, M. G., Tribulo, P., Staples, C. R., Santos, J. E. P. (2023). Dose-dependent effects of rumen-protected choline on hepatic metabolism during induction of fatty liver in dry pregnant dairy cows. PLOS ONE, 18 (10), e0290562. https://doi.org/10.1371/journal.pone.0290562
- Sánchez, V., Baumann, A., Kromm, F., Yergaliyev, T., Brandt, A., Scholda, J. et al. (2024). Oral supplementation of choline attenuates the development of alcohol-related liver disease (ALD). Molecular Medicine, 30 (1). https://doi.org/10.1186/s10020-024-00950-4
- Kansakar, U., Trimarco, V., Mone, P., Varzideh, F., Lombardi, A., Santulli, G. (2023). Choline supplements: An update. Frontiers in Endocrinology, 14. https://doi.org/10.3389/fendo.2023.1148166
- Abou-Hatab, K., Ganeshalingam, K., O’Mahony, M. S., Giurani, F., Patel, S., Woodhouse, K. (2001). The effect of community-acquired pneumonia on plasma esterases in older people. European Journal of Clinical Pharmacology, 57 (1), 55–60. https://doi.org/10.1007/s002280000259
- Razumiene, J., Leo, D., Gureviciene, V., Ratautas, D., Gaidukevic, J., Sakinyte-Urbikiene, I. (2023). L-Glutamate Biosensor for In Vitro Investigations: Application in Brain Extracts. Chemosensors, 11 (8), 418. https://doi.org/10.3390/chemosensors11080418
- Makaras, T., Razumienė, J., Gurevičienė, V., Sauliutė, G., Stankevičiūtė, M. (2022). Technical suitability and reliability of an in vivo and non-invasive biosensor-type glucose assessment as a potential biomarker for multiple stressors in fishes: an evaluation on Salmonids. Environmental Science and Pollution Research, 29 (27), 41187–41206. https://doi.org/10.1007/s11356-022-18546-y
- Stefanov, O. V. (2001). Doklinichni doslidzhennia likarskykh zasobiv. Kyiv: Avicena, 528.
- Kozhemiakin, Yu. M., Khromov, O. S., Filonenko, M. A., Saifetdinova, H. A. (2002). Naukovo-praktychni rekomendatsii z utrymannia laboratornykh tvaryn ta roboty z nymy. Kyiv: Vyd. dim «Avitsena», 153–155.
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. (Text with EEA relevance) (2010). Official Journal of the European Union, L 276, 33–79.
- Tamanoi, F. (2022). The enzymes second edition part 2. DNA Damage and Double Strand Breaks – Part B, 45–79. https://doi.org/10.1016/bs.enz.2022.10.005
- Liu, Y., Zhang, J., Lu, X., Zhang, G.-R., Qi, K., Bai, Y., Qi, W. (2022). Highly efficient electroreduction of oxygen to hydrogen peroxide on carbon catalyst via electrode-electrolyte interface engineering. Chemical Engineering Journal, 444, 136665. https://doi.org/10.1016/j.cej.2022.136665
- Sui, W., Li, W., Zhang, Z., Wu, W., Xu, Z., Wang, Y. (2023). Efficient and durable electrochemical oxygen reduction to H2O2 in acidic media assisted through catalyst layer design. Journal of Power Sources, 556, 232438. https://doi.org/10.1016/j.jpowsour.2022.232438
- Andani, A., Mellou, K., Dewda, P., Eeuwijk, J., Kassianos, G., Van Damme, P., Steffen, R. (2024). Evolution and Impact of Hepatitis a Epidemiology in Europe – Systematic Literature Review of the Last 20 Years. Journal of Viral Hepatitis, 32 (1). https://doi.org/10.1111/jvh.14030
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Julija Razumiene, Vidute Gureviciene, Ieva Sakinyte-Urbikiene, Liubov Galuzinska, Igor Seniuk, Vira Kravchenko, Dmytro Lytkin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







