Antimicrobial properties of newly synthesised aryl acyclic amino alcohols against clinical strains of enterococci
DOI:
https://doi.org/10.15587/2519-8025.2024.325076Keywords:
antibiotic resistance, Enterococcus spp., MIC, serial dilution method, antibiotics, antisepticsAbstract
Objective. The present work is devoted to the in vitro antimicrobial activity of newly synthesized aryl acyclic amino alcohols, namely, derivatives of alkyl (R-aryl) oxydialkyl ammonium salts against clinical strains of enterococci.
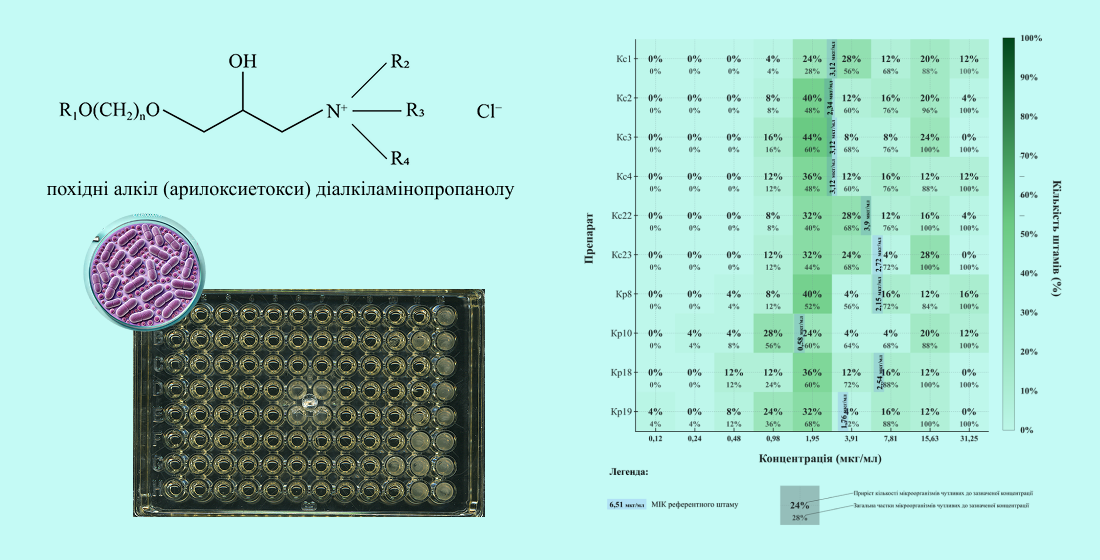
Materials and methods. The object of the study was 52 newly synthesized aryl acyclic amino alcohols, namely derivatives of quaternary salts of alkyl(aryloxyethoxy)dialkylaminopropanol, synthesized at the Institute of Organic Chemistry of the National Academy of Sciences of Ukraine. The antimicrobial activity was evaluated in vitro by agar diffusion and serial dilutions against the reference strain Enterococcus faecalis ATCC 29212 and 25 clinical isolates. The efficacy of the compounds against clinical strains was evaluated by comparison with the activity of antimicrobial drugs.
Results. As a result of the screening against the reference strain, the compounds with the most pronounced antienterococcal activity were identified. The most active ones (Kc21, Kc14, Kc28, Kc13, Kp13) had retention zones of more than 14 mm. The MIC determination identified 10 most active compounds, the MIC of Kp10 and Kp19 against the reference strain was 0.58 ± 0.10 μg/ml and 1.76 ± 0.19 μg/ml. As for clinical strains, the compounds showed moderate activity, with MIC values ranging from 0.48 to 15.63 μg/ml, inhibiting a proportion of isolates at the level of ampicillin and vancomycin. All compounds outperformed the activity of tetracycline in inhibiting antibiotic-resistant strains. The antimicrobial effect of the studied substances was at the level of decamethoxin and significantly exceeded the activity of miramistin.
Conclusions. The study made it possible to identify the 10 most active compounds against the test microorganisms and to compare them with commercial antimicrobial drugs. The list of the most effective aryl acyclic amino alcohols is presented in the following list: Kp10, Kp19, Kp8, Kc2, Kp18, Kc23 Kc1, Kc3, Kc4, and Kc22. The data obtained indicate the prospects for further study of the antimicrobial properties of alkyl (R-aryl) oxydialkyl ammonium derivatives
References
- Murray, C. J. L., Ikuta, K. S., Sharara, F., Swetschinski, L., Robles Aguilar, G., Gray, A. et al. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet, 399 (10325), 629–655. https://doi.org/10.1016/s0140-6736(21)02724-0
- WHO Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance (2024). WHO, 56. https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1
- Weiner, L. M., Webb, A. K., Limbago, B., Dudeck, M. A., Patel, J., Kallen, A. J. et al. (2016). Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infection Control & Hospital Epidemiology, 37 (11), 1288–1301. https://doi.org/10.1017/ice.2016.174
- Antimicrobial resistance surveillance in Europe (2023). ECDC. https://doi.org/10.2900/63495
- Demchenko, N., Suvorova, Z., Fedchenkova, Y., Shpychak, T., Shpychak, O., Bobkova, L., Demchenko, S. (2021). Synthesis and antibacterial activity of 3-arylaminomethyl-1-(2-oxo-2-arylethyl)-6,7,8,9-tetrahydro-5H-[1,2,4]triazolo[4,3-a] azepin-1-ium bromides and aryl-(4-R1-phenyl-5,6,7,8-tetrahydro-2,2a,8a-triazacyclopenta[cd]azulen-1-ylmethyl)-amines. ScienceRise: Pharmaceutical Science, 6 (34), 51–57. https://doi.org/10.15587/2519-4852.2021.249480
- Rosa, W. C., Rocha, I. O., Rodrigues, M. B., Coelho, H. S., Denardi, L. B., Ledur, P. C. et al. (2020). Novel Alkyl(aryl)-Substituted 2,2-Difluoro-6-(trichloromethyl)-2H-1,3,2-oxazaborinin-3-ium-2-uides: Synthesis, Antimicrobial Activity, and CT-DNA Binding Evaluations. Frontiers in Pharmacology, 11. https://doi.org/10.3389/fphar.2020.01328
- Baker, J. R., Cossar, P. J., Blaskovich, M. A. T., Elliott, A. G., Zuegg, J., Cooper, M. A. et al. (2022). Amino Alcohols as Potential Antibiotic and Antifungal Leads. Molecules, 27 (7), 2050. https://doi.org/10.3390/molecules27072050
- Dronova, M. L. (2015). Doslidzhennia vplyvu pokhidnoho arylalkilatfatyfichnykh aminospyrtiv KVM-194 na pronyknist membrany bakterii. Medychni ta farmatsevtychni nauky, 86–89.
- Korotkyi, Yu. V., Smertenko, O. A. (2013). Pat. No. 86109. Chetvertynni soli 1-[4-(1,1,3,3-tetrametylbutyl)fenoksy-1-etoksy]-3-(N-alkildialkilamino)-2-propanolu. MKP C07D 213/00. No. u201308693; declareted: 10.07.2013; published: 10.12.2013, Bul. No. 23.
- Korotkyi, Yu. V., Smertenko, O. A. (2014). Pat. No. 93482. Chetvertynni soli 1-[(2,4-dytretbutyl)fenoksy-1-etoksy]-3-(N-alkildialkilamoniiu)-2-propanolu. MKP C07D 213/00. No. u201400149; declareted: 10.01.2014; published: 10.10.2014, Bul. No. 19.
- Performance standards for antimicrobial susceptibility testing (2021). Clinical and Laboratory Standards Institute.
- Kowalska-Krochmal, B., Dudek-Wicher, R. (2021). The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens, 10 (2), 165. https://doi.org/10.3390/pathogens10020165
- Balouiri, M., Sadiki, M., Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6 (2), 71–79. https://doi.org/10.1016/j.jpha.2015.11.005
- Breakpoint tables for interpretation of MICs and zone diameters (Version 14.0) (2024). The European Committee on Antimicrobial Susceptibility Testing. Available at: http://www.eucast.org
- Sydorchuk, L. I., Sydorchuk, R. I., Mikheyev, A. O., Dzhuryak, V. S., Sydorchuk, I. Y. (2022). Selection of decamethoxine resistant variants of microorganisms while using quaternary ammonium compounds under clinical conditions for 50 years (1972-2022). Clinical and Experimental Pathology, 21 (3), 13–19. https://doi.org/10.24061/1727-4338.xxi.3.81.2022.02
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Volodymyr Nastenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







