Antimycobacterial activity of new aryloxyethoxy-dialkylaminopropanol derivatives against reference strains of nontuberculous mycobacteria
DOI:
https://doi.org/10.15587/2519-8025.2025.326435Keywords:
antimicrobial resistance, nontuberculous mycobacteria, M. smegmatis, aryl amino alcohols, MIC, proportion method, rifampicinAbstract
Objective. To evaluate the in vitro antimycobacterial activity of novel quaternary ammonium salts, derived from alkyl(aryloxyethoxy)dialkylaminopropanol against reference strains of nontuberculous mycobacteria (NTM).
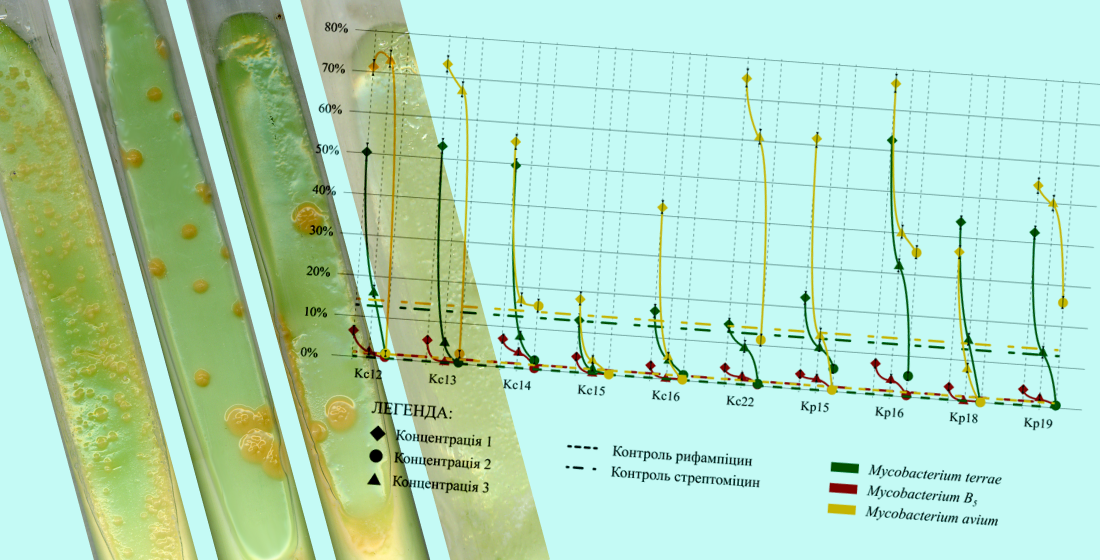
Materials and Methods. A total of 52 synthesized compounds, obtained via phase-transfer catalysis, were studied,. The activity was assessed in two stages: initial screening using the agar diffusion method (well method), followed by minimum inhibitory concentration (MIC) determination through serial dilution. Primary testing was performed on Mycobacterium smegmatis, followed by evaluation on M. terrae, M. avium, and M. B5 using the proportion method on Löwenstein–Jensen medium. Compounds were tested at three concentrations: K-I (MIC for M. smegmatis), K-II (10×MIC), and K-III (100×MIC).
Results. Ten compounds with the highest activity against M. smegmatis were identified during the screening stage. The most active was Kc12 (MIC – 0.22±0.02 µg/mL). Other active compounds included: Kc16, Kc13, Kc15, Kp15, Kp20, Kp18, Kc22, Kc14, and Kp16. Among the NTM strains, M. terrae and M. B5 were the most sensitive. At K-II, compounds Kc12, Kc13, Kc15, Kc16, Kc22, and Kp18 inhibited bacterial growth by more than 95 %. Kc15 demonstrated the efficacy comparable to rifampicin, while Kp18 and Kc12 surpassed streptomycin. M. avium exhibited resistance, with growth suppression below 5 %, observed only at K-III in a few compounds. The least active was Kp20.
Conclusions. Ten promising compounds were identified, notably Kc12, Kc15, Kp18, Kc16, and Kc22, whose activity exceeded that of streptomycin and approached rifampicin in some cases. The findings highlight the potential of these compounds for further preclinical development in the treatment of NTM infections
References
- WHO. Global tuberculosis report 2023 (2023). World Health Organization, 75. Available at: https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1
- WHO Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance (2024). WHO, 56. Available at: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1
- WHO Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis (WHO/EMP/IAU/2017.12) (2017). World Health Organization. Available at: https://iris.who.int/handle/10665/311820
- Alffenaar, J.-W., Märtson, A.-G., Heysell, S. K., Cho, J.-G., Patanwala, A., Burch, G. et al. (2021). Therapeutic Drug Monitoring in Non-Tuberculosis Mycobacteria Infections. Clinical Pharmacokinetics, 60 (6), 711–725. https://doi.org/10.1007/s40262-021-01000-6
- Gu, Y., Nie, W., Huang, H., Yu, X. (2023). Non-tuberculous mycobacterial disease: progress and advances in the development of novel candidate and repurposed drugs. Frontiers in Cellular and Infection Microbiology, 13. https://doi.org/10.3389/fcimb.2023.1243457
- Moreira, W., Lim, J. J., Yeo, S. Y., Ramanujulu, P. M., Dymock, B. W., Dick, T. (2016). Fragment-Based Whole Cell Screen Delivers Hits against M. tuberculosis and Non-tuberculous Mycobacteria. Frontiers in Microbiology, 7. https://doi.org/10.3389/fmicb.2016.01392
- Saxena, S., Spaink, H. P., Forn-Cuní, G. (2021). Drug Resistance in Nontuberculous Mycobacteria: Mechanisms and Models. Biology, 10 (2), 96. https://doi.org/10.3390/biology10020096
- Kaur, P., Krishnamurthy, R. V., Shandil, R. K., Mohan, R., & Narayanan, S. (2023). A Novel Inhibitor against the Biofilms of Non-Tuberculous Mycobacteria. Pathogens, 13 (1), 40. https://doi.org/10.3390/pathogens13010040
- Stokes, J. M., Yang, K., Swanson, K., Jin, W., Cubillos-Ruiz, A., Donghia, N. M. et al. (2020). A Deep Learning Approach to Antibiotic Discovery. Cell, 180 (4), 688-702.e13. https://doi.org/10.1016/j.cell.2020.01.021
- Osypchuk, N. О., Nastenko, V. B., Shirobokov, V. P., Korotkyi, Y. V. (2020). Sensitivity of antifungal preparations of Сandida isolates from sub-biotopes of the human oral cavity. Regulatory Mechanisms in Biosystems, 11 (1), 82–87. https://doi.org/10.15421/022011
- Nastenko, V. B., Korotkyi, Yu. V., Smertenko, O. A., Osypchuk, N. O., Shyrobokov, V. P., Chobotar, A. P. (2018). Study of antimicrobial activity of alkyl (r-aryl) oxy dialkyl ammonium salts towards the reference strains of microorganisms. Microbiology & Biotechnology, 1 (41), 18–27. https://doi.org/10.18524/2307-4663.2018.1(41).117806
- Korotkyi, Yu. V., Smertenko, O. A. (2013). Pat. No. 86109. Chetvertynni soli 1-[4-(1,1,3,3-tetrametylbutyl)fenoksy-1-etoksy]-3-(N-alkildialkilamino)-2-propanolu. MKP C07D 213/00. No. u201308693; declareted: 10.07.2013; published: 10.12.2013, Bul. No. 23.
- Korotkyi, Yu. V., Smertenko, O. A. (2014). Pat. No. 93482. Chetvertynni soli 1-[(2,4-dytretbutyl)fenoksy-1-etoksy]-3-(N-alkildialkilamoniiu)-2-propanolu. MKP C07D 213/00. No. u201400149; declareted: 10.01.2014; published: 10.10.2014, Bul. No. 19.
- Balouiri, M., Sadiki, M., Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6 (2), 71–79. https://doi.org/10.1016/j.jpha.2015.11.005
- Mpeirwe, M., Komakech, K., Ssesazi, D., Ogwang, P. E., Bazira, J. (2024). Combination Activity of Standard Antituberculosis Drugs and Extracts of Medicinal Plants Commonly Used in Traditional Treatment of Tuberculosis in Uganda. Advances in Infectious Diseases, 14 (3), 511–522. https://doi.org/10.4236/aid.2024.143037
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Volodymyr Nastenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







