Evolution of insulin production technologies: from historical discoveries of the molecule structure to modern innovations
DOI:
https://doi.org/10.15587/2519-8025.2025.349033Keywords:
Insulin, recombinant DNA technologies, biosimilars, insulin analogs, oral delivery systemsAbstract
The aim of the study is to assess the current state of the pharmaceutical market for insulins, including historical stages of studying the structure of the insulin molecule and its properties, which formed the basis for the development of commercial preparations and analogs, as well as analysis of promising biotechnological approaches to improve the treatment of diabetes mellitus (DM).
Materials and methods. The materials used were scientific publications, official websites of manufacturing companies, FDA and EMA databases, clinical trial registries. Methods of content analysis, comparative, analytical, and generalization of information were applied.
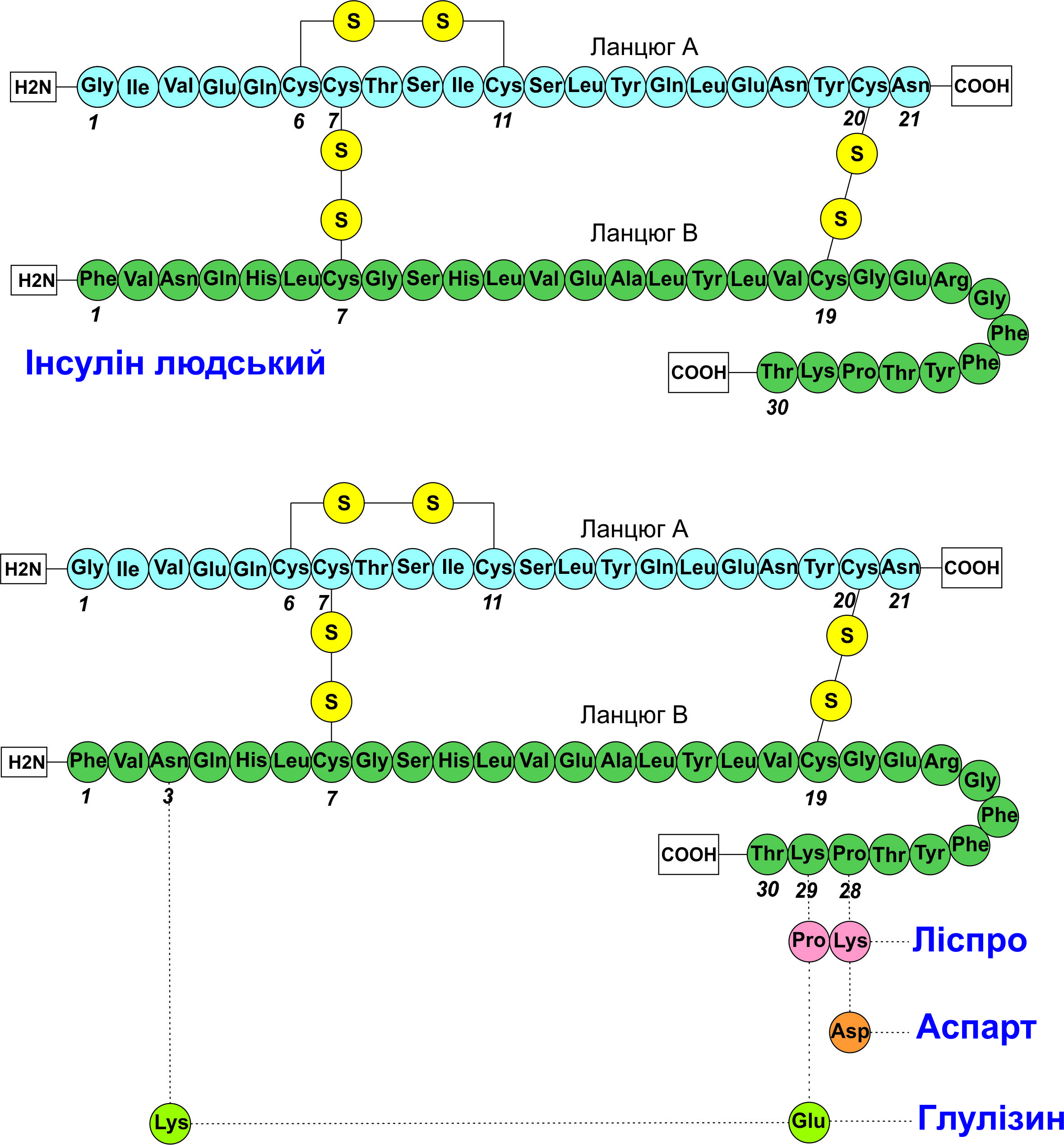
Results and discussion. The results indicate that recombinant insulin preparations (from rapid-acting analogs to long-acting ones) provide better glycemic control but are limited by high development and production costs. Innovations include combined preparations with GLP-1 agonists, glucose-sensitive insulins, and oral forms, which face bioavailability challenges.
Conclusions. The analysis points to the evolution of insulin production technologies from determining the molecule structure and implementing recombinant DNA technologies, which enabled the transition to human recombinant preparations and analogs. The market offers preparations with various profiles (from ultra-rapid to ultra-long), including biphasic mixtures, improving glycemic control. Combinations of insulin with GLP-1 agonists, amylin analogs (pramlintide), and the development of glucose-sensitive insulins have potential for personalized therapy but are limited by technical challenges (stability, biocompatibility). Oral forms face low bioavailability, but the use of nanotechnology and effective excipients opens prospects for improving accessibility and effectiveness of DM treatment
References
- International Diabetes Federation. Available at: https://diabetesatlas.org/
- Pro zatverdzhennia Poriadku provedennia klinichnykh vyprobuvan likarskykh zasobiv ta ekspertyzy materialiv klinichnykh vyprobuvan i Typovoho polozhennia pro komisii z pytan etyky: Nakaz Ministerstva okhorony zdorovia Ukrainy No. 690. 23.09.2009. Availabe at: https://moz.gov.ua/uk/u-piv-miljona-ukrainciv-diagnostovano-cukrovij-diabet-u-2023-roci
- Kasinathan, D., Guo, Z., Sarver, D. C., Wong, G. W., Yun, S., Michels, A. W. et al. (2024). Cell-Surface ZnT8 Antibody Prevents and Reverses Autoimmune Diabetes in Mice. Diabetes, 73 (5), 806–818. https://doi.org/10.2337/db23-0568
- Baker, D. E. (2023). Teplizumab. Hospital Pharmacy, 58 (6), 549–556. https://doi.org/10.1177/00185787231160431
- Monzani, P. S., Sangalli, J. R., Sampaio, R. V., Guemra, S., Zanin, R., Adona, P. R. et al. (2024). Human proinsulin production in the milk of transgenic cattle. Biotechnology Journal, 19 (3). https://doi.org/10.1002/biot.202300307
- Beck, R. W., Bailey, R. J., Klein, K. R., Aleppo, G., Levy, C. J., Diner, J. et al. (2025). Inhaled Technosphere Insulin Plus Insulin Degludec for Adults with Type 1 Diabetes: The INHALE-3 Extension Study. Diabetes Technology & Therapeutics, 27 (3), 170–178. https://doi.org/10.1089/dia.2024.0582
- Gaddas, M., Saida, I. B., Saad, H. B. (2025). Twenty years of inhaled insulin: promise, setbacks, and future directions. EXCLI Journal, 28 (24), 573–577. https://doi.org/10.17179/excli2025-8260
- Silva, I. B. B., Kimura, C. H., Colantoni, V. P., Sogayar, M. C. (2022). Stem cells differentiation into insulin-producing cells (IPCs): recent advances and current challenges. Stem Cell Research & Therapy, 13 (1). https://doi.org/10.1186/s13287-022-02977-y
- Kumar, D., Tanwar, R. (2024). World’s first: stem cell therapy reverses diabetes. Stem Cell Research & Therapy, 15 (1). https://doi.org/10.1186/s13287-024-04036-0
- Zhang, T., Tang, J. Z., Fei, X., Li, Y., Song, Y., Qian, Z., Peng, Q. (2021). Can nanoparticles and nano‒protein interactions bring a bright future for insulin delivery? Acta Pharmaceutica Sinica B, 11 (3), 651–667. https://doi.org/10.1016/j.apsb.2020.08.016
- Kaliuzhnaia, O. S., Khokhlenkova, N. V., Panenko, M. V. (2024). The analysis of recombinant insulin production technologies. News of Pharmacy, 108 (2), 25–36. https://doi.org/10.24959/nphj.24.158
- Alyas, Ej., Rafiq, A., Amir, H., Khan, S. U., Sultana, T., Ali, A., Hameed, A., Ahmad, I., Kazmi, A., Sajid, T., Ahmad, A. (2021). Human Insulin: History, Recent Advances, and Expression Systems for Mass Production. Biomedical Research and Therapy, 8 (9), 4540–4561. https://doi.org/10.15419/bmrat.v8i9.692
- Artasensi, A., Pedretti, A., Vistoli, G., Fumagalli, L. (2020). Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules, 25 (8), 1987. https://doi.org/10.3390/molecules25081987
- Brown, H., Sanger, F., Kitai, R. (1955). The structure of pig and sheep insulins. Biochemical Journal, 60 (4), 556–565. https://doi.org/10.1042/bj0600556
- Stretton, A. O. W. (2002). The First Sequence: Fred Sanger and Insulin. Genetics, 162 (2), 527–532. https://doi.org/10.1093/genetics/162.2.527
- Halban, P. A. (1991). Structural domains and molecular lifestyles of insulin and its precursors in the pancreatic Beta cell. Diabetologia, 34 (11), 767–778. https://doi.org/10.1007/bf00408349
- Steiner, D. F., James, D. E. (1992). Cellular and molecular biology of the Beta cell. Diabetologia, 35 (S2), S41–S48. https://doi.org/10.1007/bf00586278
- Weiss, M., Steiner, D. F., Philipson, L. H.; Feingold, K. R., Ahmed, S. F., Anawalt, B. et al. (Eds.) (2014). Insulin biosynthesis, secretion, structure, and structure-activity relationships. Endotext. South Dartmouth: MDText.com, Inc. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279029/
- Saisho, Y. (2016). Postprandial C-Peptide to Glucose Ratio as a Marker of β Cell Function: Implication for the Management of Type 2 Diabetes. International Journal of Molecular Sciences, 17 (5), 744. https://doi.org/10.3390/ijms17050744
- Szukiewicz, D. (2023). Molecular Mechanisms for the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity. International Journal of Molecular Sciences, 24 (12), 9818. https://doi.org/10.3390/ijms24129818
- Gorai, B., Vashisth, H. (2022). Progress in Simulation Studies of Insulin Structure and Function. Frontiers in Endocrinology, 13. https://doi.org/10.3389/fendo.2022.908724
- Galloway, J., Chance, R. (1994). Improving Insulin Therapy: Achievements and Challenges. Hormone and Metabolic Research, 26 (12), 591–598. https://doi.org/10.1055/s-2007-1001766
- Bußmann, A. B., Grünerbel, L. M., Durasiewicz, C. P., Thalhofer, T. A., Wille, A., Richter, M. (2021). Microdosing for drug delivery application – A review. Sensors and Actuators A: Physical, 330, 112820. https://doi.org/10.1016/j.sna.2021.112820
- Giugliano, D., Scappaticcio, L., Longo, M., Caruso, P., Maiorino, M. I., Bellastella, G., Esposito, K. (2021). Simplification of complex insulin therapy: a story of dogma and therapeutic resignation. Diabetes Research and Clinical Practice, 178, 108958. https://doi.org/10.1016/j.diabres.2021.108958
- Brange, J., Ribel, U., Hansen, J. F., Dodson, G., Hansen, M. T., Havelund, S. et al. (1988). Monomeric insulins obtained by protein engineering and their medical implications. Nature, 333 (6174), 679–682. https://doi.org/10.1038/333679a0
- Bakaysa, D. L., Radziuk, J., Havel, H. A., Brader, M. L., Li, S., Dodd, S. W. et al. (1996). Physicochemical basis for the rapid time‐action of LysB28ProB29‐insulin: Dissociation of a protein‐ligand complex. Protein Science, 5 (12), 2521–2531. https://doi.org/10.1002/pro.5560051215
- Birnbaum, D. T., Kilcomons, M. A., DeFelippis, M. R., Beals, J. M. (1997). Assembly and Dissociation of Human Insulin and LysB28ProB29-Insulin Hexamers: A Comparison Study. Pharmaceutical Research, 14 (1), 25–36. https://doi.org/10.1023/a:1012095115151
- Purple Book Database of Licensed Biological Products: U.S. Food and Drug Administration. Available at: https://purplebooksearch.fda.gov/
- Download medicine data (2024). European Medicines Agency. Available at: https://www.ema.europa.eu/en/medicines/download-medicine-data
- Adams, M. J., Blundell, T. L., Dodson, E. J., Dodson, G. G., Vijayan, M., Baker, E. N. et al. (1969). Structure of Rhombohedral 2 Zinc Insulin Crystals. Nature, 224 (5218), 491–495. https://doi.org/10.1038/224491a0
- Pekar, A. H., Frank, B. H. (1972). Conformation of proinsulin. Comparison of insulin and proinsulin self-association at neutral pH. Biochemistry, 11(22), 4013–4016. https://doi.org/10.1021/bi00772a001
- Owens, D. R. (2011). Insulin Preparations with Prolonged Effect. Diabetes Technology & Therapeutics, 13 (S1), S-5–S-14. https://doi.org/10.1089/dia.2011.0068
- Berenson, D. F., Weiss, A. R., Wan, Z., Weiss, M. A. (2011). Insulin analogs for the treatment of diabetes mellitus: therapeutic applications of protein engineering. Annals of the New York Academy of Sciences, 1243 (1), 40–54. https://doi.org/10.1111/j.1749-6632.2012.06468.x
- Maxwell, L. C., Bischoff, F. (1935). Augmentation of the physiologic response to insulin. American Journal of Physiology-Legacy Content, 112 (1), 172–175. https://doi.org/10.1152/ajplegacy.1935.112.1.172
- Scott, D. A., Fisher, A. M. (1935). The effect of zinc salts on the action of insulin. The Journal of Pharmacology and Experimental Therapeutics, 55 (2), 206–221. https://doi.org/10.1016/s0022-3565(25)04174-6
- Blundell, T. L., Dodson, G. G., Dodson, E., Hodgkin, D. C., Vijayan, M. (1971). X-Ray Analysis and the Structure of Insulin. Proceedings of the 1970 Laurentian Hormone Conference, 27, 1–40. https://doi.org/10.1016/b978-0-12-571127-2.50025-0
- Hodgkin, D. C. (1971). X rays and the structures of insulin. British Medical Journal, 4 (5785), 447–451. https://doi.org/10.1136/bmj.4.5785.447
- Jarosinski, M. A., Dhayalan, B., Chen, Y.-S., Chatterjee, D., Varas, N., Weiss, M. A. (2021). Structural principles of insulin formulation and analog design: A century of innovation. Molecular Metabolism, 52, 101325. https://doi.org/10.1016/j.molmet.2021.101325
- Phillips, N. B., Wan, Z., Whittaker, L., Hu, S.-Q., Huang, K., Hua, Q. et al. (2010). Supramolecular Protein Engineering. Journal of Biological Chemistry, 285 (16), 11755–11759. https://doi.org/10.1074/jbc.c110.105825
- Vashisth, H. (2015). Theoretical and Computational Studies of Peptides and Receptors of the Insulin Family. Membranes, 5 (1), 48–83. https://doi.org/10.3390/membranes5010048
- Brader, M. L., Kaarsholm, N. C., Dunn, M. F. (1990). The R-state proinsulin and insulin hexamers mimic the carbonic anhydrase active site. Journal of Biological Chemistry, 265 (26), 15666–15670. https://doi.org/10.1016/s0021-9258(18)55450-8
- Rahuel-Clermont, S., French, C. A., Kaarsholm, N. C., Dunn, M. F. (1997). Mechanisms of Stabilization of the Insulin Hexamer through Allosteric Ligand Interactions. Biochemistry, 36 (19), 5837–5845. https://doi.org/10.1021/bi963038q
- Kosinová, L., Veverka, V., Novotná, P., Collinsová, M., Urbanová, M., Moody, N. R. et al. (2014). Insight into the Structural and Biological Relevance of the T/R Transition of the N-Terminus of the B-Chain in Human Insulin. Biochemistry, 53 (21), 3392–3402. https://doi.org/10.1021/bi500073z
- Blader, M. L., Dunn, M. F. (1991). Insulin hexamers: new conformations and applications. Trends in Biochemical Sciences, 16, 341–345. https://doi.org/10.1016/0968-0004(91)90140-q
- Roy, M., Brader, M. L., Lee, R. W., Kaarsholm, N. C., Hansen, J. F., Dunn, M. F. (1989). Spectroscopic signatures of the T to R conformational transition in the insulin hexamer. Journal of Biological Chemistry, 264 (32), 19081–19085. https://doi.org/10.1016/s0021-9258(19)47269-4
- Berchtold, H., Hilgenfeld, R. (1999). Binding of phenol to R6 insulin hexamers. Biopolymers, 51 (2), 165–172. https://doi.org/10.1002/(sici)1097-0282(1999)51:2<165::aid-bip6>3.0.co;2-x
- Hallas-Møller, K., Petersen, K., Schlichtkrull, J. (1952). Crystalline and Amorphous Insulin-Zinc Compounds with Prolonged Action. Science, 116 (3015), 394–398. https://doi.org/10.1126/science.116.3015.394
- Hallas-Mø, K. (1956). The Lente Insulins. Diabetes, 5 (1), 7–14. https://doi.org/10.2337/diab.5.1.7
- Schlichtkrull, J., Munck, O., Jersild, M. (1965). Insulin Rapitard and Insulin Actrapid. Acta Medica Scandinavica, 177 (1), 103–113. https://doi.org/10.1111/j.0954-6820.1965.tb01811.x
- Humulin® Therapies. Eli Lilly and Company. Available at: https://medical.lilly.com/us/products/medical-information/diabetes/humulin
- Our medicines. Diabetes medications. Novo Nordisk. Available at: https://www.novonordisk.com/our-products/our-medicines.html
- Vetsulin. Merck Animal Health. Available at: https://www.merck-animal-health-usa.com/pet-owners/vetsulin/
- Bentley, G., Dodson, G., Lewitova, A. (1978). Rhombohedral insulin crystal transformation. Journal of Molecular Biology, 126 (4), 871–875. https://doi.org/10.1016/0022-2836(78)90026-8
- Derewenda, U., Derewenda, Z., Dodson, E. J., Dodson, G. G., Reynolds, C. D., Smith, G. D. et al. (1989). Phenol stabilizes more helix in a new symmetrical zinc insulin hexamer. Nature, 338 (6216), 594–596. https://doi.org/10.1038/338594a0
- Hagedorn, H. C., Jensen, B. N., Krarup, N. B., Wodstrup, I. (1936). Protamine Insulinate. Journal of the American Medical Association, 106 (3), 177–180. https://doi.org/10.1001/jama.1936.02770030007002
- Scott, D. A., Fisher, A. M. (1936). Studies on insulin with protamine. The Journal of Pharmacology and Experimental Therapeutics, 58 (1), 78–92. https://doi.org/10.1016/s0022-3565(25)09793-9
- Yip, C. M., Brader, M. L., Frank, B. H., DeFelippis, M. R., Ward, M. D. (2000). Structural Studies of a Crystalline Insulin Analog Complex with Protamine by Atomic Force Microscopy. Biophysical Journal, 78 (1), 466–473. https://doi.org/10.1016/s0006-3495(00)76609-4
- Kurtzhals, P., Havelund, S., Jonassen, I., Kiehr, B., Larsen, U. D., Ribel, U., Markussen, J. (1995). Albumin binding of insulins acylated with fatty acids: characterization of the ligand-protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochemical Journal, 312 (3), 725–731. https://doi.org/10.1042/bj3120725
- Peters, T. (1985). Serum Albumin. Advances in Protein Chemistry, 37, 161–245. https://doi.org/10.1016/s0065-3233(08)60065-0
- Kragh-Hansen, U. (1990). Structure and ligand binding properties of human serum albumin. Danish Medical Bulletin, 37 (1), 57–84.
- Wasko, J., Wolszczak, M., Zajaczkowska, Z., Dudek, M., Kolesinska, B. (2024). Human serum albumin as a potential drug delivery system for N-methylated hot spot insulin analogs inhibiting hormone aggregation. Bioorganic Chemistry, 143, 107104. https://doi.org/10.1016/j.bioorg.2024.107104
- Rao, S. S., Somayaji, Y., Kulal, A. (2022). Synthesis and Evaluation of the Insulin-Albumin Conjugate with Prolonged Glycemic Control. ACS Omega, 7 (6), 5131–5138. https://doi.org/10.1021/acsomega.1c06119
- Whittingham, J. L., Havelund, S., Jonassen, I. (1997). Crystal Structure of a Prolonged-Acting Insulin with Albumin-Binding Properties. Biochemistry, 36 (10), 2826–2831. https://doi.org/10.1021/bi9625105
- Kaarsholm, N. C., Havelund, S., Hougaard, P. (1990). Ionization behavior of native and mutant insulins: pK perturbation of B13-Glu in aggregated species. Archives of Biochemistry and Biophysics, 283 (2), 496–502. https://doi.org/10.1016/0003-9861(90)90673-m
- Rosskamp, R. H., Park, G. (1999). Long-acting insulin analogs. Diabetes Care, 22 (2), 109–113.
- Campbell, R. K., White, J. R., Levien, T., Baker, D. (2001). Insulin glargine. Clinical Therapeutics, 23 (12), 1938–1957. https://doi.org/10.1016/s0149-2918(01)80148-x
- Toujeo®. Sanofi. Available at: https://www.toujeopro.com/
- Kim, A. P., Bindler, R. J. (2016). The Future of Biosimilar Insulins. Diabetes Spectrum, 29 (3), 161–166. https://doi.org/10.2337/diaspect.29.3.161
- BLA vs. NDA: Understanding the Differences in Biopharmaceutical Approvals. Syner G. Available at: https://synergbiopharma.com/blog/bla-vs-nda/
- Guidance document. Applications Covered by Section 505(b)(2) (1999). U.S. Food and Drug Administration, FDA. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/applications-covered-section-505b2
- Industry Information and Guidance (2025). Biosimilars: U.S. Food and Drug Administration, FDA. Available at: https://www.fda.gov/drugs/biosimilars/industry-information-and-guidance
- Approved Biosimilars. U.S. Food and Drug Administration, FDA. Available at: https://biosimilarsforum.org/approved-biosimilars/
- Yu, M., Zhang, C., Xu, H., Dong, Y., Zhu, H., Xia, C., Feng, J. (2025). Design of a novel long-acting insulin analogs by acetylation modification and compared with insulin Icodec. Scientific Reports, 15 (1). https://doi.org/10.1038/s41598-025-94014-0
- Barlocco, D. (2003). Insulin detemir. Novo Nordisk. Current Opinion Investigational Drugs, 4 (4), 449–454.
- Vasselli, J. R., Pi-Sunyer, F. X., Wall, D. G., John, C. S., Chapman, C. D., Currie, P. J. (2017). Central effects of insulin detemir on feeding, body weight, and metabolism in rats. American Journal of Physiology-Endocrinology and Metabolism, 313 (5), E613–E621. https://doi.org/10.1152/ajpendo.00111.2016
- Kurtzhals, P., Schäffer, L., Sørensen, A., Kristensen, C., Jonassen, I., Schmid, C., Trüb, T. (2000). Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes, 49 (6), 999–1005. https://doi.org/10.2337/diabetes.49.6.999
- Hordern, S. V. M., Wright, J. E., Umpleby, A. M., Shojaee-Moradie, F., Amiss, J., Russell-Jones, D. L. (2005). Comparison of the effects on glucose and lipid metabolism of equipotent doses of insulin detemir and NPH insulin with a 16-h euglycaemic clamp. Diabetologia, 48 (3), 420–426. https://doi.org/10.1007/s00125-005-1670-1
- Rossetti, P., Porcellati, F., Ricci, N. B., Candeloro, P., Cioli, P., Bolli, G. B., Fanelli, C. G. (2008). Different Brain Responses to Hypoglycemia Induced by Equipotent Doses of the Long-Acting Insulin Analog Detemir and Human Regular Insulin in Humans. Diabetes, 57 (3), 746–756. https://doi.org/10.2337/db07-1433
- Hermansen, K., Davies, M., Derezinski, T., Martinez Ravn, G., Clauson, P., Home, P. (2006). A 26-Week, Randomized, Parallel, Treat-to-Target Trial Comparing Insulin Detemir With NPH Insulin as Add-On Therapy to Oral Glucose-Lowering Drugs in Insulin-Naïve People With Type 2 Diabetes. Diabetes Care, 29 (6), 1269–1274. https://doi.org/10.2337/dc05-1365
- Rosenstock, J., Davies, M., Home, P. D., Larsen, J., Koenen, C., Schernthaner, G. (2008). A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia, 51 (3), 408–416. https://doi.org/10.1007/s00125-007-0911-x
- Steensgaard, D. B., Schluckebier, G., Strauss, H. M., Norrman, M., Thomsen, J. K., Friderichsen, A. V. et al. (2013). Ligand-Controlled Assembly of Hexamers, Dihexamers, and Linear Multihexamer Structures by the Engineered Acylated Insulin Degludec. Biochemistry, 52 (2), 295–309. https://doi.org/10.1021/bi3008609
- Tambascia, M. A., Eliaschewitz, F. G. (2015). Degludec: the new ultra-long insulin analogue. Diabetology & Metabolic Syndrome, 7 (1). https://doi.org/10.1186/s13098-015-0037-0
- Jonassen, I., Havelund, S., Hoeg-Jensen, T., Steensgaard, D. B., Wahlund, P.-O., Ribel, U. (2012). Design of the Novel Protraction Mechanism of Insulin Degludec, an Ultra-long-Acting Basal Insulin. Pharmaceutical Research, 29 (8), 2104–2114. https://doi.org/10.1007/s11095-012-0739-z
- Nauck, M. A., Quast, D. R., Wefers, J., Meier, J. J. (2021). GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Molecular Metabolism, 46, 101102. https://doi.org/10.1016/j.molmet.2020.101102
- Nishimura, E., Pridal, L., Glendorf, T., Hansen, B. F., Hubálek, F., Kjeldsen, T. et al. (2021). Molecular and pharmacological characterization of insulin icodec: a new basal insulin analog designed for once-weekly dosing. BMJ Open Diabetes Research & Care, 9 (1), e002301. https://doi.org/10.1136/bmjdrc-2021-002301
- Goldman, J., Triplitt, C., Isaacs, D. (2024). Icodec: A Novel Once-Weekly Basal Insulin for Diabetes Management. Annals of Pharmacotherapy, 59 (6), 554–569. https://doi.org/10.1177/10600280241287790
- Ashraf, T., Kumar, A., Tara, A., Memon, N., Muhammad, A., Turesh, M. et al. (2025). Once-weekly insulin icodec vs. daily insulin glargine in type 2 diabetes: a meta-analysis with longitudinal insights. Annals of Medicine & Surgery, 87 (7), 4452–4466. https://doi.org/10.1097/ms9.0000000000003392
- Awiqli insulin icodec. European Medicines Agency. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/awiqli
- Novo Nordisk Resubmits FDA Application for First Once-Weekly Insulin for Type 2 Diabetes. MedPath. Available at: https://trial.medpath.com/news/08cba5b13b946426/novo-nordisk-resubmits-fda-application-for-first-once-weekly-insulin-for-type-2-diabetes
- Slieker, L. J., Brooke, G. S., DiMarchi, R. D., Flora, D. B., Green, J. S., Hoffmann, J. A. et al. (1997). Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia, 40, 54–61. https://doi.org/10.1007/s001250051402
- Kesavadev, J., Basanth, A., Shankar, A., Saboo, B., Mohan, A. R., Joshi, S. et al. (2025). An Overview of Currently Available Injectable Therapies in Diabetes: A Guide to Practitioners. Advances in Therapy, 42 (8), 3634–3656. https://doi.org/10.1007/s12325-025-03250-3
- Raedler, L. A. (2016). Tresiba (Insulin Degludec Injection) and Ryzodeg 70/30 (Insulin Degludec and Insulin Aspart Injection): Two New Insulin Analogs for Glycemic Control in Diabetes Mellitus. American health & drug benefits, 9, 144–148. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5013846/
- Becker, R. H. A., Frick, A. D. (2008). Clinical Pharmacokinetics and Pharmacodynamics of Insulin Glulisine. Clinical Pharmacokinetics, 47 (1), 7–20. https://doi.org/10.2165/00003088-200847010-00002
- Lih, A., Hibbert, E., Wong, T., Girgis, Ch. M., Garg, N., Carter, J. N. (2010). The role of insulin glulisine to improve glycemic control in children with diabetes mellitus. Diabetes, metabolic syndrome and obesity: targets and therapy, 26 (3), 403–412. https://doi.org/10.2147/dmsott.s5116
- Heise, T., Hövelmann, U., Zijlstra, E., Stender-Petersen, K., Jacobsen, J. B., & Haahr, H. (2016). A Comparison of Pharmacokinetic and Pharmacodynamic Properties Between Faster-Acting Insulin Aspart and Insulin Aspart in Elderly Subjects with Type 1 Diabetes Mellitus. Drugs & Aging, 34 (1), 29–38. https://doi.org/10.1007/s40266-016-0418-6
- Shah, H. K., Shah, M., Patel, H., Maslekar, A., Kanzariya, T. (2025). Comparison of Two Short-acting Insulin Formulations in the Management of Blood Glucose in Undergoing Coronary Artery Bypass Graft Surgery Patients. International Journal of Diabetes and Technology, 4 (2), 29–34. https://doi.org/10.4103/ijdt.ijdt_2_25
- Dutta, D., Mohindra, R., Mahajan, K., Sharma, M. (2023). Performance of Fast-Acting Aspart Insulin as Compared to Aspart Insulin in Insulin Pump for Managing Type 1 Diabetes Mellitus: A Meta-Analysis. Diabetes & Metabolism Journal, 47 (1), 72–81. https://doi.org/10.4093/dmj.2022.0035
- Leohr, J., Dellva, M. A., LaBell, E., Coutant, D. E., Arrubla, J., Plum‐Mörschel, L. et al. (2023). Ultra rapid lispro (Lyumjev®) shortens time to recovery from hyperglycaemia compared to Humalog® in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes, Obesity and Metabolism, 26 (1), 215–223. https://doi.org/10.1111/dom.15307
- Giorgino, F., Battelino, T., Bergenstal, R. M., Forst, T., Green, J. B., Mathieu, C. et al. (2023). The Role of Ultra-Rapid-Acting Insulin Analogs in Diabetes: An Expert Consensus. Journal of Diabetes Science and Technology, 19 (2), 452–469. https://doi.org/10.1177/19322968231204584
- Bailey, C. J., Flatt, P. R., Conlon, J. M. (2025). Multifunctional incretin peptides in therapies for type 2 diabetes, obesity and associated co-morbidities. Peptides, 187, 171380. https://doi.org/10.1016/j.peptides.2025.171380
- Leading drugs worldwide based on projected 2025 sales (2024). Statista. Available at: https://www.statista.com/statistics/973523/top-drugs-by-year-on-year-sales-increase/?srsltid=AfmBOoqM8YOOnt-ZldMq1TtqMzA5Tlqhp0gTTqh7uxBbHbj7WbB_Bn66
- Malone, E. (2025). Top 10 Drugs Q2 2025: Mounjaro Overtakes Ozempic: Citeline. Available at: https://insights.citeline.com/scrip/business/top-10-drugs-q2-2025-mounjaro-overtakes-ozempic-HJVMVTVQPFHC7NR7XUR46WEZBQ/
- ElSayed, N. A., McCoy, R. G., Aleppo, G., Bajaj, M., Balapattabi, K., Beverly, E. A. et al. (2024). 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes – 2025. Diabetes Care, 48 (1), S181–S206. https://doi.org/10.2337/dc25-s009
- Jung, H. N., Cho, Y. K., Min, S. H., Kim, H. S., Kim, Y.-J., Park, J.-Y. et al. (2022). Free Versus Fixed-Ratio Combination of Basal Insulin and GLP-1 Receptor Agonists in Type 2 Diabetes Uncontrolled With GLP-1 Receptor Agonists: A Systematic Review and Indirect Treatment Comparison. Frontiers in Endocrinology, 13. https://doi.org/10.3389/fendo.2022.870722
- Candido, R., Nicolucci, A., Larosa, M., Rossi, M. C., Napoli, R., Gabellieri, E. et al. (2024). Treatment intensification following glucagon-like peptide-1 receptor agonist in type 2 diabetes: Comparative effectiveness analyses between free vs. fixed combination of GLP-1 RA and basal insulin. RESTORE-G real-world study. Nutrition, Metabolism and Cardiovascular Diseases, 34 (8), 1846–1853. https://doi.org/10.1016/j.numecd.2024.03.023
- Ahmed, A., Monir. Akl, M. (2024). Exploring a Synergistic Approach: Dual GLP-1 Agonist Combined with Degludec Basal Insulin for Early Type 1 Diabetes Treatment and its Impact on Albumin-Insulin Producing Cells Expression. Advanced Pharmaceutical Bulletin, 14 (2), 262–265. https://doi.org/10.34172/apb.2024.040
- Bolli, G. B., Porcellati, F., Lucidi, P., Fanelli, C. G., Perseghin, G., Horowitz, M. et al. (2025). An overview of randomized clinical trials of fixed-ratio combinations of basal insulin plus GLP-1RA (injectable therapy): Lessons for advancing therapy in people with type 2 diabetes. Diabetes, Obesity and Metabolism, 27 (7), 14–25. https://doi.org/10.1111/dom.16616
- Derzhavnyi reiestr likarskykh zasobiv Ukrainy. Available at: http://www.drlz.com.ua/
- Hoogwerf, B. J., Doshi, K. B., Diab, D. (2008). Pramlintide, the synthetic analogue of amylin: physiology, pathophysiology, and effects on glycemic control, body weight, and selected biomarkers of vascular risk. Vascular health and risk management, 4 (2), 355–362. https://doi.org/10.2147/vhrm.s1978
- Sinézia, C., Sisnande, T., Icart, L. P., Lima, L. M. T. R. (2024). Oral delivery of the amylin receptor agonist pramlintide. Peptide Science, 116 (4). https://doi.org/10.1002/pep2.24346
- Riddle, M. C., Nahra, R., Han, J., Castle, J., Hanavan, K., Hompesch, M. et al. (2018). Control of Postprandial Hyperglycemia in Type 1 Diabetes by 24-Hour Fixed-Dose Coadministration of Pramlintide and Regular Human Insulin: A Randomized, Two-Way Crossover Study. Diabetes Care, 41 (11), 2346–2352. https://doi.org/10.2337/dc18-1091
- Riddle, M. C. (2020). Rediscovery of the Second β-Cell Hormone: Co-replacement With Pramlintide and Insulin in Type 1 Diabetes. Diabetes Care, 43 (3), 518–521. https://doi.org/10.2337/dci19-0077
- Haidar, A., Tsoukas, M. A., Bernier-Twardy, S., Yale, J.-F., Rutkowski, J., Bossy, A. et al. (2020). A Novel Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care, 43 (3), 597–606. https://doi.org/10.2337/dc19-1922
- Maikawa, C. L., Chen, P. C., Vuong, E. T., Nguyen, L. T., Mann, J. L., d’Aquino, A. I. et al. (2021). Ultra‐Fast Insulin–Pramlintide Co‐Formulation for Improved Glucose Management in Diabetic Rats. Advanced Science, 8 (21). https://doi.org/10.1002/advs.202101575
- Kommera, S. P., Kumar, A., Chitkara, D., Mittal, A. (2024). Pramlintide an Adjunct to Insulin Therapy: Challenges and Recent Progress in Delivery. The Journal of Pharmacology and Experimental Therapeutics, 388 (1), 81–90. https://doi.org/10.1124/jpet.123.001679
- Rege, N. K., Phillips, N. F. B., Weiss, M. A. (2017). Development of glucose-responsive ‘smart’ insulin systems. Current Opinion in Endocrinology, Diabetes & Obesity, 24 (4), 267–278. https://doi.org/10.1097/med.0000000000000345
- Brouillard, J. E. (2025). Update on the status of Glucose-Responsive Insulins. Clinical Diabetes, 43 (2), 322–323. https://doi.org/10.2337/cd25-0007
- FDA Approves Medtronic MiniMed 780G System (2023). Danatech. Diabetes Technology ADCES. Available at: https://www.adces.org/education/danatech/latest-news/danatech-latest-news/2023/11/16/fda-approved-medtronic-minimed-780g-system
- Studies show promising results for individuals with type 2 diabetes and young children with type 1 diabetes on MiniMed™ 780G system (2025). Medtronic. Available at: https://news.medtronic.com/2025-06-20-Studies-show-promising-results-for-individuals-with-type-2-diabetes-and-young-children-with-type-1-diabetes-on-MiniMed-TM-780G-system
- Safety evaluation of an advanced Hybrid Closed Loop System using Lyumjev with the Tandem t:Slim X2 Insulin pump with control-iq technology in adults, adolescents and children with type 1 diabetes (2024). ClinicalTrials, National Library of Medicine. Available at: https://www.clinicaltrials.gov/study/NCT05403502
- Tandem Diabetes Care Announces t:slim X2™ Insulin Pump Compatibility with Abbott’s FreeStyle Libre® 3 Plus Sensor in the United States (2025). Tandem Diabetes Care. Available at: https://investor.tandemdiabetes.com/news-releases/news-release-details/tandem-diabetes-care-announces-tslim-x2tm-insulin-pump
- Ji, K., Wei, X., Kahkoska, A. R., Zhang, J., Zhang, Y., Xu, J. et al. (2024). An orally administered glucose-responsive polymeric complex for high-efficiency and safe delivery of insulin in mice and pigs. Nature Nanotechnology, 19 (12), 1880–1891. https://doi.org/10.1038/s41565-024-01764-5
- Chou, D. H.-C., Webber, M. J., Tang, B. C., Lin, A. B., Thapa, L. S., Deng, D. et al. (2015). Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proceedings of the National Academy of Sciences, 112 (8), 2401–2406. https://doi.org/10.1073/pnas.1424684112
- Liu, W., Zhang, J., Wang, Y., He, Y., Wang, Y., Wei, X. et al. (2025). Long-acting glucose-responsive insulin with swift onset-of-action. Journal of Controlled Release, 383, 113826. https://doi.org/10.1016/j.jconrel.2025.113826
- Kruse, Th., Kofoed-Hansen, M., Muenzel, M. W. B., Thoegersen, H., Sauerberg, P., Rasmussen, J. E. et al. (2020). Glucose-sensitive albumin-binding derivatives. Patent US20200325160A1.
- Varas, N., Jarosinski, M. A., Chen, Y.-S., Ni, C.-L., Grabowski, R. A., Tai, N. et al. (2025). Ultrastable Insulin-Glucagon Fusion Protein Exploits an Endogenous Hepatic Switch to Mitigate Hypoglycemic Risk. ACS Pharmacology & Translational Science, 8 (9), 3240–3258. https://doi.org/10.1021/acsptsci.5c00362
- Bode, B. W., Boyd, J., Shah, A., Parkes, D., Ghosh, S., Cherrington, A. D. (2020). 7-LB: Insulin and Glucagon Coadministration in Type 1 Diabetes Prevents Hypoglycemia without Worsening Hyperglycemia. Diabetes, 69 (1). https://doi.org/10.2337/db20-7-lb
- Pedersen, C., Bouman, S. D., Porsgaard, T., Rosenkilde, M. M., Roed, N. K. (2018). Dual treatment with a fixed ratio of glucagon and insulin increases the therapeutic window of insulin in diabetic rats. Physiological Reports, 6 (6), e13657. https://doi.org/10.14814/phy2.13657
- Hoeg-Jensen, T., Kruse, T., Brand, C. L., Sturis, J., Fledelius, C., Nielsen, P. K. et al. (2024). Glucose-sensitive insulin with attenuation of hypoglycaemia. Nature, 634 (8035), 944–951. https://doi.org/10.1038/s41586-024-08042-3
- Hoeg-Jensen, T. (2021). Review: Glucose-sensitive insulin. Molecular Metabolism, 46, 101107. https://doi.org/10.1016/j.molmet.2020.101107
- Asare-Bediako, I., Paszkiewicz, R. L., Kim, S. P., Woolcott, O. O., Kolka, C. M., Burch, M. A. et al. (2018). Variability of Directly Measured First-Pass Hepatic Insulin Extraction and Its Association With Insulin Sensitivity and Plasma Insulin. Diabetes, 67(8), 1495–1503. https://doi.org/10.2337/db17-1520
- Roger, R. C. (2025). Oral Insulin – Harnessing the Natural Physiology of Glucose Control in the Body. Medical Research Archives, 13 (1). https://doi.org/10.18103/mra.v13i1.6180
- Wong, C. Y., Martinez, J., Dass, C. R. (2016). Oral delivery of insulin for treatment of diabetes: status quo, challenges and opportunities. Journal of Pharmacy and Pharmacology, 68 (9), 1093–1108. https://doi.org/10.1111/jphp.12607
- Fontana, G., Innamorati, G., Giacomello, L. (2025). Nanoparticle-Based Oral Insulin Delivery: Challenges, Advances, and Future Directions. Pharmaceutics, 17 (12), 1563. https://doi.org/10.3390/pharmaceutics17121563
- Zhang, E., Zhu, H., Song, B., Shi, Y., Cao, Z. (2024). Recent advances in oral insulin delivery technologies. Journal of Controlled Release, 366, 221–230. https://doi.org/10.1016/j.jconrel.2023.12.045
- Rekha, M. R., Sharma, C. P. (2009). Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. Journal of Controlled Release, 135 (2), 144–151. https://doi.org/10.1016/j.jconrel.2009.01.011
- Shams K. (2015). Nanoencapsulation of insulin using blends of biodegradable polymers and in vitro controlled release of insulin. Journal of Chemical Engineering & Process Technology, 06 (02). https://doi.org/10.4172/2157-7048.1000228
- Zhou, J., Zhang, J., Sun, Y., Luo, F., Guan, M., Ma, H. et al. (2023). A nano-delivery system based on preventing degradation and promoting absorption to improve the oral bioavailability of insulin. International Journal of Biological Macromolecules, 244, 125263. https://doi.org/10.1016/j.ijbiomac.2023.125263
- Ghassemi, A. H., van Steenbergen, M. J., Talsma, H., van Nostrum, C. F., Jiskoot, W., Crommelin, D. J. A., Hennink, W. E. (2009). Preparation and characterization of protein loaded microspheres based on a hydroxylated aliphatic polyester, poly(lactic-co-hydroxymethyl glycolic acid). Journal of Controlled Release, 138 (1), 57–63. https://doi.org/10.1016/j.jconrel.2009.04.025
- Zhang, Y., Wu, X., Meng, L., Zhang, Y., Ai, R., Qi, N. et al. (2012). Thiolated Eudragit nanoparticles for oral insulin delivery: Preparation, characterization and in vivo evaluation. International Journal of Pharmaceutics, 436 (1-2), 341–350. https://doi.org/10.1016/j.ijpharm.2012.06.054
- Lin, C.-H., Chen, C.-H., Lin, Z.-C., Fang, J.-Y. (2017). Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. Journal of Food and Drug Analysis, 25 (2), 219–234. https://doi.org/10.1016/j.jfda.2017.02.001
- Muntoni, E., Marini, E., Ahmadi, N., Milla, P., Ghè, C., Bargoni, A. et al. (2019). Lipid nanoparticles as vehicles for oral delivery of insulin and insulin analogs: preliminary ex vivo and in vivo studies. Acta Diabetologica, 56 (12), 1283–1292. https://doi.org/10.1007/s00592-019-01403-9
- Zhang, Y., Xiong, M., Ni, X., Wang, J., Rong, H., Su, Y. et al. (2021). Virus-Mimicking Mesoporous Silica Nanoparticles with an Electrically Neutral and Hydrophilic Surface to Improve the Oral Absorption of Insulin by Breaking Through Dual Barriers of the Mucus Layer and the Intestinal Epithelium. ACS Applied Materials & Interfaces, 13 (15), 18077–18088. https://doi.org/10.1021/acsami.1c00580
- Scudeller, L. A., Mavropoulos, E., Tanaka, M. N., Costa, A. M., Braga, C. A. C., López, E. O. et al. (2017). Effects on insulin adsorption due to zinc and strontium substitution in hydroxyapatite. Materials Science and Engineering: C, 79, 802–811. https://doi.org/10.1016/j.msec.2017.05.061
- Zou, J.-J., Wei, G., Xiong, C., Yu, Y., Li, S., Hu, L. et al. (2022). Efficient oral insulin delivery enabled by transferrin-coated acid-resistant metal-organic framework nanoparticles. Science Advances, 8 (8). https://doi.org/10.1126/sciadv.abm4677
- Maher, S., Brayden, D. J., Casettari, L., Illum, L. (2019). Application of Permeation Enhancers in Oral Delivery of Macromolecules: An Update. Pharmaceutics, 11 (1), 41. https://doi.org/10.3390/pharmaceutics11010041
- Wang, J., Kong, M., Zhou, Z., Yan, D., Yu, X., Cheng, X. et al. (2017). Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydrate Polymers, 157, 596–602. https://doi.org/10.1016/j.carbpol.2016.10.021
- Raptis, K., Heade, J., Cunha, C., van de Weert, M., Saaby, L., Rønholt, S., Nielsen, H. M. (2025). Permeation Enhancer‐based Ionogel Shows Remarkable Potential for Oral Insulin Delivery. Advanced Healthcare Materials, 14 (20). https://doi.org/10.1002/adhm.202500946
- Maher, S., Heade, J., McCartney, F., Waters, S., Bleiel, S. B., Brayden, D. J. (2018). Effects of surfactant-based permeation enhancers on mannitol permeability, histology, and electrogenic ion transport responses in excised rat colonic mucosae. International Journal of Pharmaceutics, 539 (1-2), 11–22. https://doi.org/10.1016/j.ijpharm.2018.01.008
- Perinelli, D. R., Cespi, M., Casettari, L., Vllasaliu, D., Cangiotti, M., Ottaviani, M. F. et al. (2016). Correlation among chemical structure, surface properties and cytotoxicity of N-acyl alanine and serine surfactants. European Journal of Pharmaceutics and Biopharmaceutics, 109, 93–102. https://doi.org/10.1016/j.ejpb.2016.09.015
- Fan, W., Xia, D., Zhu, Q., Li, X., He, S., Zhu, C. et al. (2018). Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials, 151, 13–23. https://doi.org/10.1016/j.biomaterials.2017.10.022
- Wu, H., Nan, J., Yang, L., Park, H. J., Li, J. (2023). Insulin-loaded liposomes packaged in alginate hydrogels promote the oral bioavailability of insulin. Journal of Controlled Release, 353, 51–62. https://doi.org/10.1016/j.jconrel.2022.11.032
- Kumar, P., Kaur, N., Tiwari, P., Gupta, A. K., Mobin, S. M. (2023). Gelatin-Coated Copper-Based Metal–Organic Framework for Controlled Insulin Delivery: Possibility toward Oral Delivery System. ACS Materials Letters, 5 (4), 1100–1108. https://doi.org/10.1021/acsmaterialslett.2c01175
- Gu, Zh., Yu, J. (2018). Glucose-responsive insulin delivery microneedle system. Patent US11191815B2.
- Aich, K., Singh, T., Dang, S. (2022). Advances in microneedle-based transdermal delivery for drugs and peptides. Drug Delivery and Translational Research, 12 (7), 1556–1568. https://doi.org/10.1007/s13346-021-01056-8
- Oramed Completes Patient Enrollment in Pivotal Phase 3 Oral Insulin Study ORA-D-013-1 (2022). ANP. Available at: https://persportaal.anp.nl/artikel/CSN-030522109/oramed-completes-patient-enrollment-in-pivotal-phase-3-oral-insulin-study-ora-d-013-1
- Eldor, R., Francis, B. H., Fleming, A., Neutel, J., Homer, K., Kidron, M. et al. (2022). Oral insulin (ORMD-0801) in type 2 diabetes mellitus: A dose-finding 12-week randomized placebo-controlled study, Diabetes, Obesity and Metabolism, 25 (4), 943–952. https://doi.org/10.1111/dom.14901
- Oramed Announces Top-line Results from Phase 3 Trial of ORMD-0801 for the Treatment of Type 2 Diabetes (2023). Oramed. Available at: https://oramed.com/oramed-announces-top-line-results-from-phase-3-trial-of-ormd-0801-for-the-treatment-of-type-2-diabetes/
- Halberg, I. B., Lyby, K., Wassermann, K., Heise, T., Zijlstra, E., Plum-Mörschel, L. (2019). Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. The Lancet Diabetes & Endocrinology, 7 (3), 179–188. https://doi.org/10.1016/s2213-8587(18)30372-3
- National Library of Medicine. Available at: https://clinicaltrials.gov/
- EudraCT. Available at: https://eudract.ema.europa.eu/
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Olha Kaliuzhnaia, Natalya Khokhlenkova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







