Clinical, hematological, and immunological aspects of chronic myeloid leukemia: from molecular mechanisms to functional recovery
DOI:
https://doi.org/10.15587/2519-4798.2025.340270Keywords:
chronic myeloid leukemia, CD26 leukemic stem cells, additional ASXL1/RUNX1/TP53 mutations, extracellular vesicles, ferroptosis, natural killer cells, PD-1, NKG2A-HLA-E, tyrosine kinase inhibitors, remission without treatmentAbstract
The aim. To systematize and critically analyze data on clinical, hematological, molecular, and immunological determinants of chronic myeloid leukemia (CML) progression and treatment-free remission (TFR) with an emphasis on the pathophysiological mechanisms of leukemic stem cells (LSCs, CD26+), additional mutations (ASXL1, RUNX1, TP53), extracellular vesicles (EVs), ferroptosis, and immune control axes (PD-1/PD-L1, NKG2A-HLA-E), as well as the immunomodulatory effects of tyrosine kinase inhibitors (TKIs).
Materials and methods. A targeted narrative review of guidelines (ELN, NCCN) and peer-reviewed publications from 2000–2025 from PubMed/PMC and specialized journals was conducted. Clinical predictors of response, pathophysiological mechanisms of resistance, and immune biomarkers of TFR success were summarized; a conceptual synthesis of evidence on the impact of different generations of TKIs on the immune landscape was performed, taking into account the post-COVID context.
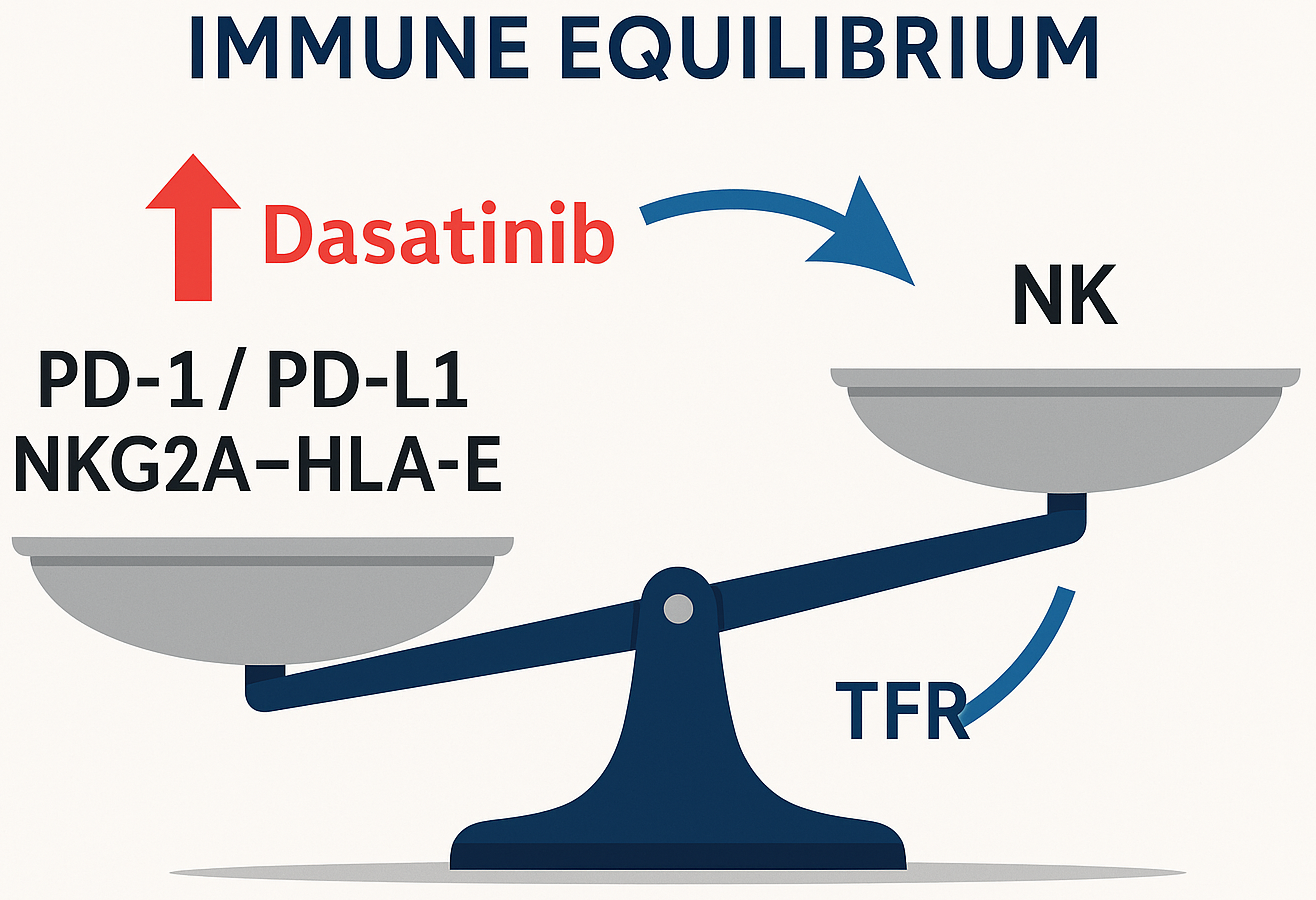
Results. CD26+ LSCs determine the early dynamics of the molecular response to ITC, but their persistence during TFR is not a sufficient predictor of relapse, emphasizing the leading role of immune surveillance. Additional ASXL1/RUNX1/TP53 mutations are associated with worse event-free survival and the need for early intensification of the strategy. EVs carry oncogenic signals (in particular, BCR-ABL1 transcript) and form an immunosuppressive microenvironment. Imatinib-resistant cell models show increased sensitivity to ferroptosis inducers, opening up a new therapeutic vector. Successful TFR is associated with higher numbers and maturity of NK cells (CD56+ CD16+ CD57+), the presence of memory-like NK (NKG2C+), high perforin content in NK and innate CD8+ T cells, and simultaneously low PD-1 expression. The NKG2A-HLA-E axis acts as an additional “brake” on cytotoxicity; its blockade potentially synergizes with anti-PD-1. Dasatinib, unlike imatinib/nilotinib, partially relieves inhibition via NKG2A, enhancing NK cytotoxicity. The COVID-19 pandemic has changed patients' immune “fingerprints” (fewer NK cells with higher activation), which should be taken into account when selecting for TFR.
Conclusions. Control of CML is a balance between deep cytoreduction with ITC and competent immune surveillance. Personalized tactics should combine: early identification of high-risk mutations, targeting CD26+ LSC, modification of EV signalling and induction of ferroptosis, as well as immunotherapeutic combinations (PD-1/PD-L1 and/or NKG2A-HLA-E blockade), which can increase the proportion of patients eligible for sustained TFR and bring the concept of functional recovery closer
References
- Sicuranza, A., Cavalleri, A., Bernardi, S. (2025). The biology of chronic myeloid leukemia: an overview of the new insights and biomarkers. Frontiers in Oncology, 15. https://doi.org/10.3389/fonc.2025.1546813
- Hochhaus, A., Baccarani, M., Silver, R. T., Schiffer, C., Apperley, J. F., Cervantes, F. et al. (2020). European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia, 34 (4), 966–984. https://doi.org/10.1038/s41375-020-0776-2
- Shah, N. P., Bhatia, R., Altman, J. K., Amaya, M., Begna, K. H., Berman, E. et al. (2024). Chronic Myeloid Leukemia, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 22 (1), 43–69. https://doi.org/10.6004/jnccn.2024.0007
- Cross, N. C. P., Ernst, T., Branford, S., Cayuela, J.-M., Deininger, M., Fabarius, A. et al. (2023). European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia, 37 (11), 2150–2167. https://doi.org/10.1038/s41375-023-02048-y
- Kwaśnik, P., Giannopoulos, K. (2021). Treatment-Free Remission – A New Aim in the Treatment of Chronic Myeloid Leukemia. Journal of Personalized Medicine, 11 (8), 697. https://doi.org/10.3390/jpm11080697
- Zhong, F., Zhang, X., Wang, Z., Li, X., Huang, B., Kong, G., Wang, X. (2024). The therapeutic and biomarker significance of ferroptosis in chronic myeloid leukemia. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1402669
- Sanchez, M. B., Vasconcelos Cordoba, B., Pavlovsky, C., Moiraghi, B., Varela, A., Custidiano, R. et al. (2023). In-depth characterization of NK cell markers from CML patients who discontinued tyrosine kinase inhibitor therapy. Frontiers in Immunology, 14. https://doi.org/10.3389/fimmu.2023.1241600
- Garcia, C. A. B., Binelli, L. S., Palma, L. C., Marani, L. O., Medeiros, M., Carvalho, A. G. De et al. (2024). Immune Content of Mature and Licensed Cytotoxic Cells Is Associated with Treatment-Free Remission in Chronic Myeloid Leukemia. Blood, 144, 1756–1756. https://doi.org/10.1182/blood-2024-208109
- Decroos, A., Meddour, S., Demoy, M., Piccirilli, N., Rousselot, P., Nicolini, F. E. et al. (2024). The CML experience to elucidate the role of innate T-cells as effectors in the control of residual cancer cells and as potential targets for cancer therapy. Frontiers in Immunology, 15. https://doi.org/10.3389/fimmu.2024.1473139
- Li, Y., Li, Z., Tang, Y., Zhuang, X., Feng, W., Boor, P. P. C. et al. (2024). Unlocking the therapeutic potential of the NKG2A-HLA-E immune checkpoint pathway in T cells and NK cells for cancer immunotherapy. Journal for ImmunoTherapy of Cancer, 12 (10), e009934. https://doi.org/10.1136/jitc-2024-009934
- Radich, J. P., Wall, M., Branford, S., Campbell, C. D., Chaturvedi, S., DeAngelo, D. J. et al. (2023). Molecular response in newly diagnosed chronic-phase chronic myeloid leukemia: prediction modeling and pathway analysis. Haematologica, 108 (6), 1567–1578. https://doi.org/10.3324/haematol.2022.281878
- Sanchez, M. B., Cordoba, B. V., Pavlovsky, C., Moiraghi, B., Varela, A. I., Giere, I. et al. (2025). The Influence of the COVID-19 Pandemic in NK Cell Subpopulations from CML Patients Enrolled in the Argentina Stop Trial. Cells, 14 (9), 628. https://doi.org/10.3390/cells14090628
- Nasnas, P. E., Jabbour, E. J., Sasaki, K., Issa, G. C., Masarova, L., Short, N. J., Haddad, F. G. (2024). Failure of Treatment-Free Remission after a Prolonged Deep Molecular Response in Patients with Chronic Myeloid Leukemia. Acta Haematologica, 148 (1), 105–110. Portico. https://doi.org/10.1159/000538651
- Coyle, C., Ma, M., Abraham, Y., Mahony, C. B., Steel, K., Simpson, C. et al. (2024). NK cell subsets define sustained remission in rheumatoid arthritis. JCI Insight, 9 (23). https://doi.org/10.1172/jci.insight.182390
- Camacho, M. F., Peña, M., Toloza, M. J., Moiraghi, B., Enrico, A., Mariano, R. et al. (2025). Evaluation of leukemic stem cell (CD26 +) in chronic myeloid leukemia patients with different molecular responses and in treatment-free remission. Clinical and Experimental Medicine, 25 (1). https://doi.org/10.1007/s10238-025-01626-x
- Wood, E. K., Reid, B. M., Sheerar, D. S., Donzella, B., Gunnar, M. R., Coe, C. L. (2024). Lingering Effects of Early Institutional Rearing and Cytomegalovirus Infection on the Natural Killer Cell Repertoire of Adopted Adolescents. Biomolecules, 14 (4), 456. https://doi.org/10.3390/biom14040456
- Kuznetsova, V., Krishnan, V., Costa, A., Ren, X., Ricketts, T. D., Patel, S. B. et al. (2025). Chronic inflammation deters natural killer cell fitness and cytotoxicity in myeloid leukemia. Blood Advances, 9 (4), 759–773. https://doi.org/10.1182/bloodadvances.2024014592
- Zhang, L., Zhao, Y., Dong, Y., Jiang, X. (2025). NK cell-based immunotherapy strategies for myeloid leukemia. Frontiers in Immunology, 16. https://doi.org/10.3389/fimmu.2025.1621885
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Olena Kucher

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.








