Atrial fibrillation in coronary artery disease patients: gut microbiota composition and echocardiography indexes
DOI:
https://doi.org/10.15587/2519-4798.2023.297055Keywords:
coronary artery disease, atrial fibrillation, echocardiography, gut microbiota compositionAbstract
The aim: to find connections between gut microbiota composition and transthoracic echocardiography (TTE) indexes in patients with coronary artery disease (CAD) and atrial fibrillation (AF).
Materials and methods: 300 patients were divided into 3 groups: first (CAD) – 149 patients with CAD but without arrhythmias; second (CAD+AF) – 124 patients with CAD and AF paroxysm; and the control group – 27 patients without CAD and arrhythmias. 16-S rRNA sequencing checked gut microbiota composition. TTE was done by ALOKA SSD-5000.
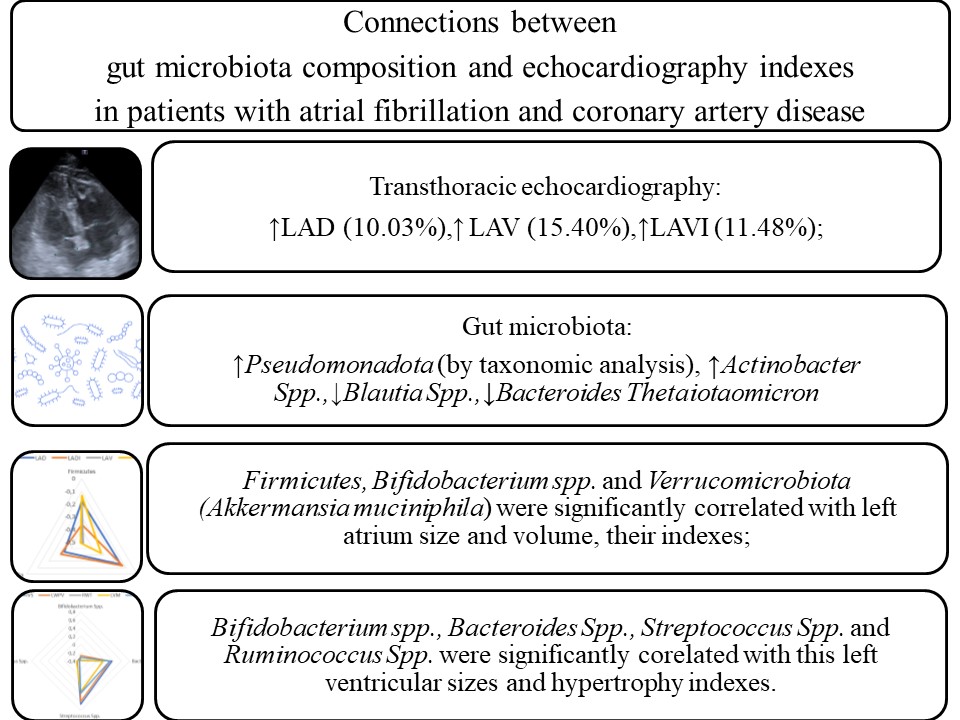
Results: The II group patients were characterized by the increase of LAD (10.03 %), LAV (15.40 %) and LAVI (11.48 %) in comparison with the I group, P<0.05. The II group patients were characterized by a rise of Pseudomonadota in comparison with the I group, P<0.05. Also, II group patients were characterized by rise of Actinobacter Spp. and decrease of Blautia Spp., Bacteroides Thetaiotaomicron in comparison with the I group, P<0.05. Firmicutes were correlated with AO (r=0.308), LADI (r=-0.363), RV (r=-0.470), IVS (r=-0.381), LVPW (r=-0.345), LVM (r=-0.476) and EF (r=0,312), P<0.05. Akkermansia Muciniphila was correlated with LAD (r=-0.343), LADI (r=-0.308), LAV (r=-0.494), LAVI (r=-0.488), RAV (r=-0.316), RAVI (r=-0.397), RV (r=-0.383), EF (r=0.332), P<0.05. Bifidobacterium Spp. were correlated with LAV (r=-0.487), LAVI (r=-0.327), RV (r=-0.341), IVS (r=-0.306), RWT (r=-0.389), LVM (r=-0.369), LVMI (r=-0.312), EF (r=0.317), P<0.05. Streptococcus Spp. were correlated with AO (r=0,329), LVOT (r=0,390), RV (r=0,393), IVS (r=0,648), LVPW (r=0,579), RWT (r=0,356), LVM (r=0,336), LVMI (r=0,376), P<0.05. Ruminococcus Spp. were correlated with AO (r=0,412), LVOT (r=0,351), LADI (r=-0.343), IVS (r=-0.316), LVPW (r=-0.367), LVM (r=-0.302), LVMI (r=-0.379), P<0.05.
Conclusion: Gut microbiota composition and TTE indexes play a significant role in CAD and AF pathogenesis. Firmicutes, Bifidobacterium spp., and Verrucomicrobiota (Akkermansia muciniphila) were significantly correlated with left atrium size and volume, as well as their ultrasound indexes. Bifidobacterium spp., Bacteroides Spp., Streptococcus Spp. and Ruminococcus Spp. were significantly correlated with left ventricular sizes and its hypertrophy indexes
References
- Hindricks, G., Potpara, T., Dagres, N., Arbelo, E., Bax, J. J., Blomström-Lundqvist, C. et al. (2020). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal, 42 (5), 373–498. doi: https://doi.org/10.1093/eurheartj/ehaa612
- Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E., Funck-Brentano, C. et al. (2019). 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal, 41 (3), 407–477. doi: https://doi.org/10.1093/eurheartj/ehz425
- Gawałko, M., Agbaedeng, T. A., Saljic, A., Müller, D. N., Wilck, N., Schnabel, R. et al. (2021). Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovascular Research, 118 (11), 2415–2427. doi: https://doi.org/10.1093/cvr/cvab292
- Schoeler, M., Caesar, R. (2019). Dietary lipids, gut microbiota and lipid metabolism. Reviews in Endocrine and Metabolic Disorders, 20 (4), 461–472. doi: https://doi.org/10.1007/s11154-019-09512-0
- Patterson, E., Ryan, P. M., Cryan, J. F., Dinan, T. G., Ross, R. P., Fitzgerald, G. F., Stanton, C. (2016). Gut microbiota, obesity and diabetes. Postgraduate Medical Journal, 92 (1087), 286–300. doi: https://doi.org/10.1136/postgradmedj-2015-133285
- Ling, Z., Liu, X., Cheng, Y., Yan, X., Wu, S. (2020). Gut microbiota and aging. Critical Reviews in Food Science and Nutrition, 62 (13), 3509–3534. doi: https://doi.org/10.1080/10408398.2020.1867054
- Efremova, I., Maslennikov, R., Poluektova, E., Zharkova, M., Kudryavtseva, A., Krasnov, G. et al. (2023). Gut Dysbiosis and Hemodynamic Changes as Links of the Pathogenesis of Complications of Cirrhosis. Microorganisms, 11 (9), 2202. doi: https://doi.org/10.3390/microorganisms11092202
- Zuo, K., Fang, C., Liu, Z., Fu, Y., Liu, Y., Liu, L., Wang, Y. et al. (2022). Commensal microbe-derived SCFA alleviates atrial fibrillation via GPR43/NLRP3 signaling. International Journal of Biological Sciences, 18 (10), 4219–4232. doi: https://doi.org/10.7150/ijbs.70644
- Chen, K., Zheng, X., Feng, M., Li, D., Zhang, H. (2017). Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Frontiers in Physiology, 8. doi: https://doi.org/10.3389/fphys.2017.00139
- Ahmad, A. F., Caparrós-Martin, J. A., Gray, N., Lodge, S., Wist, J., Lee, S., O’Gara, F., Shah, A., Ward, N. C., Dwivedi, G. (2023). Insights into the associations between the gut microbiome, its metabolites, and heart failure. American Journal of Physiology-Heart and Circulatory Physiology, 325 (6), H1325–H1336. doi: https://doi.org/10.1152/ajpheart.00436.2023
- McDonagh, T. A., Metra, M., Adamo, M., Gardner, R. S., Baumbach, A., Böhm, M. et al. (2023). 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal, 44 (37), 3627–3639. doi: https://doi.org/10.1093/eurheartj/ehad195
- Mitchell, C., Rahko, P. S., Blauwet, L. A., Canaday, B., Finstuen, J. A., Foster, M. C. et al. (2019). Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. Journal of the American Society of Echocardiography, 32 (1), 1–64. doi: https://doi.org/10.1016/j.echo.2018.06.004
- Faizi, N., Alvi, Y. (2023). Biostatistics Manual for Health Research. Elsevier. doi: https://doi.org/10.1016/c2022-0-00374-3
- Tufano, A., Galderisi, M. (2020). Can echocardiography improve the prediction of thromboembolic risk in atrial fibrillation? Evidences and perspectives. Internal and Emergency Medicine, 15 (6), 935–943. doi: https://doi.org/10.1007/s11739-020-02303-5
- Magne, F., Gotteland, M., Gauthier, L., Zazueta, A., Pesoa, S., Navarrete, P., Balamurugan, R. (2020). The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients, 12 (5), 1474. doi: https://doi.org/10.3390/nu12051474
- Bahar-Tokman, H., Demirci, M., Keskin, F., Cagatay, P., Taner, Z., Ozturk-Bakar, Y. et al. (2022). Firmicutes/Bacteroidetes Ratio in the Gut Microbiota and IL-1β, IL-6, IL-8, TLR2, TLR4, TLR5 Gene Expressions in Type 2 Diabetes. Clinical Laboratory, 68 (09/2022). doi: https://doi.org/10.7754/clin.lab.2022.211244
- Amin, N., Liu, J., Bonnechere, B., MahmoudianDehkordi, S., Arnold, M., Batra, R. et al. (2023). Interplay of Metabolome and Gut Microbiome in Individuals With Major Depressive Disorder vs Control Individuals. JAMA Psychiatry, 80 (6), 597–609. doi: https://doi.org/10.1001/jamapsychiatry.2023.0685
- Kasahara, K., Kerby, R. L., Zhang, Q., Pradhan, M., Mehrabian, M., Lusis, A. J., Bergström, G., Bäckhed, F., Rey, F. E. (2023). Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host & Microbe, 31 (6), 1038–1053.e10. doi: https://doi.org/10.1016/j.chom.2023.05.011
- Nendl, A., Raju, S. C., Broch, K., Mayerhofer, C. C. K., Holm, K., Halvorsen, B. et al. (2023). Intestinal fatty acid binding protein is associated with cardiac function and gut dysbiosis in chronic heart failure. Frontiers in Cardiovascular Medicine, 10. doi: https://doi.org/10.3389/fcvm.2023.1160030
- Tsai, H.-J., Tsai, W.-C., Hung, W.-C., Hung, W.-W., Chang, C.-C., Dai, C.-Y., Tsai, Y.-C. (2021). Gut Microbiota and Subclinical Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Nutrients, 13 (8), 2679. doi: https://doi.org/10.3390/nu13082679
- Luo, Y., Zhang, Y., Han, X., Yuan, Y., Zhou, Y., Gao, Y. et al. (2022). Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine, 82, 104087. doi: https://doi.org/10.1016/j.ebiom.2022.104087S
- Sharma, R. K., Yang, T., Oliveira, A. C., Lobaton, G. O., Aquino, V., Kim, S. et al. (2019). Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circulation Research, 124 (5), 727–736. doi: https://doi.org/10.1161/circresaha.118.313882
- Anderson, G., Mazzoccoli, G. (2019). Left Ventricular Hypertrophy: Roles of Mitochondria CYP1B1 and Melatonergic Pathways in Co-Ordinating Wider Pathophysiology. International Journal of Molecular Sciences, 20 (16), 4068. doi: https://doi.org/10.3390/ijms20164068
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Iryna Melnychuk, Maryna Sharayeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.








