Effects of different therapies on the lipid profile in children with juvenile idiopathic arthritis
DOI:
https://doi.org/10.15587/2519-4798.2025.343791Keywords:
juvenile idiopathic arthritis, dyslipidemia, lipid profile, adalimumab, tocilizumab, methotrexate, atherogenic coefficient, glucocorticoids, lipid paradox, JADAS27Abstract
The aim of the study was to carry out a comparative effect assessment of methotrexate (MTX), adalimumab (ADA), and tocilizumab (TOC) on the blood lipid profile in children with JIA to figure out possible metabolic consequences and risks.
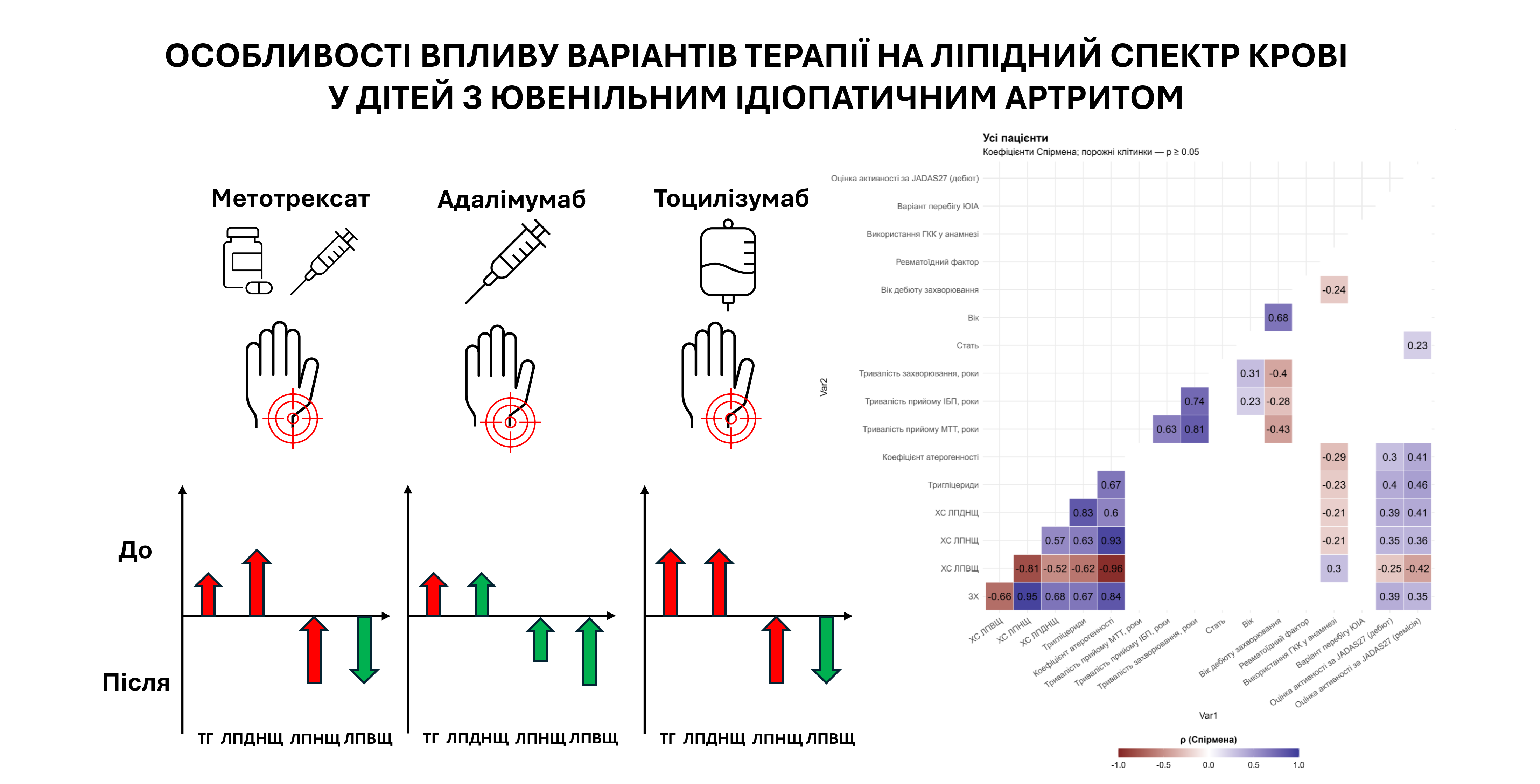
Materials and methods: 120 patients with JIA were enrolled in the study and later divided into 3 different therapy-based groups: ADA group (n=60), TOC group (n=30), and MTX group (n=30). All patients underwent the same clinical, laboratory and instrumental evaluation, which included the disease activity assessment by JADAS27 scale and lipid profile analysis (total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), very low density lipoprotein cholesterol (VLDL-C), triglycerides (TG), atherogenic coefficient (AC), both at disease onset and 3 months after reaching the pharmacological remission.
Results: There were no statistically significant differences in TC, VLDL-C, and AC levels between the therapy groups at enrollment. However, the TOC group had higher TG and VLDL-C levels. All groups showed decreases in LDL-C and HDL-C levels, therefore suggesting a “lipid paradox”. Statistically significant differences were detected during remission: patients in the ADA group had a stable lipid profile with increasing HDL-C levels, whereas in the TOC and MTX groups a significant increase in atherogenic lipid parameters was seen (TC, LDL-C, TG, AC) together with a reduction in HDL-C levels. The relative incidence of dyslipidemia in remission was 33% in the ADA group, 97% in the TOC group, and 73% in the MTX group. Only in ADA group, 8.3% of patients showed regression of dyslipidemia. Spearman correlation analysis showed direct relations of JIA activity by JADAS27 with atherogenic lipids, confirming the inflammation influence on lipid metabolism even in remission.
Conclusions: The results suggest that the use of different JIA therapy can significantly affect the blood lipid profile. TNF-α inhibitors helped to stabilize or even improve lipid profiles, while the therapy with tocilizumab and methotrexate was associated with the development of atherogenic dyslipidemia. This confirms the need for lipid profile monitoring in children with JIA
References
- Martini, A., Lovell, D. J., Albani, S., Brunner, H. I., Hyrich, K. L., Thompson, S. D., Ruperto, N. (2022). Juvenile idiopathic arthritis. Nature Reviews Disease Primers, 8 (1). https://doi.org/10.1038/s41572-021-00332-8
- Zhao, W.-J., Deng, J.-H., Li, C.-F. (2023). Lipid profiles in patients with juvenile idiopathic arthritis: a systematic literature review and meta-analysis. Lipids in Health and Disease, 22 (1). https://doi.org/10.1186/s12944-023-01885-1
- Drosos, A. A., Venetsanopoulou, A. A., Pelechas, E., Voulgari, P. V. (2024). Exploring Cardiovascular Risk Factors and Atherosclerosis in Rheumatoid Arthritis. European Journal of Internal Medicine, 128, 1–9. https://doi.org/10.1016/j.ejim.2024.07.016
- Aranda-Valera, I. C., Arias de la Rosa, I., Roldán-Molina, R., Ábalos-Aguilera, M. del C., Torres-Granados, C., Patiño-Trives, A. et al. (2020). Subclinical cardiovascular risk signs in adults with juvenile idiopathic arthritis in sustained remission. Pediatric Rheumatology, 18 (1). https://doi.org/10.1186/s12969-020-00448-3
- Ringold, S., Angeles‐Han, S. T., Beukelman, T., Lovell, D., Cuello, C. A., Becker, M. L. et al. (2019). 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non‐Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care & Research, 71 (6), 717–734. https://doi.org/10.1002/acr.23870
- Horneff, G., Minden, K., Rolland, C., Daly, A. C. H., Borlenghi, C., Ruperto, N. (2023). Efficacy and safety of TNF inhibitors in the treatment of juvenile idiopathic arthritis: a systematic literature review. Pediatric Rheumatology, 21 (1). https://doi.org/10.1186/s12969-023-00798-8
- Fragoulis, G. E., Soulaidopoulos, S., Sfikakis, P. P., Dimitroulas, T., Kitas, G. D. (2021). Effect of Biologics on Cardiovascular Inflammation: Mechanistic Insights and Risk Reduction. Journal of Inflammation Research, 14, 1915–1931. https://doi.org/10.2147/jir.s282691
- Petty, R. E., Southwood, T. R., Manners, P., Baum, J., Glass, D. N., Goldenberg, J. et al. (2004). International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. The Journal of Rheumatology, 31 (2), 390–392.
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report (2011). Pediatrics, 128 (5), S213–S256. https://doi.org/10.1542/peds.2009-2107c
- Fragoulis, G. E., Panayotidis, I., Nikiphorou, E. (2020). Cardiovascular Risk in Rheumatoid Arthritis and Mechanistic Links: From Pathophysiology to Treatment. Current Vascular Pharmacology, 18 (5), 431–446. https://doi.org/10.2174/1570161117666190619143842
- Rigby, W. F. C., Lampl, K., Low, J. M., Furst, D. E. (2017). Review of Routine Laboratory Monitoring for Patients with Rheumatoid Arthritis Receiving Biologic or Nonbiologic DMARDs. International Journal of Rheumatology, 2017, 1–15. https://doi.org/10.1155/2017/9614241
- Nasef, S. I., Abouzied, S. S. I. M., Elfiky, S. M., Zeiton, A. (2021). P008 Lipid profile disorders in children and adolescents with Juvenile Idiopathic Arthritis. Rheumatology, 60 (5). https://doi.org/10.1093/rheumatology/keab722
- Luo, Y., Ren, X., Weng, S., Yan, C., Mao, Q., Peng, D. (2021). Improvements in High-Density Lipoprotein Quantity and Quality Contribute to the Cardiovascular Benefits by Anti-tumor Necrosis Factor Therapies in Rheumatoid Arthritis: A Systemic Review and Meta-Analysis. Frontiers in Cardiovascular Medicine, 8. https://doi.org/10.3389/fcvm.2021.765749
- Jia, X., Yang, Z., Li, J., Mei, Z., Jia, L., Yan, C. (2024). The impact of biologic agents on cardiovascular risk factors in patients with rheumatoid arthritis: A meta analysis. PLOS ONE, 19 (8), e0306513. https://doi.org/10.1371/journal.pone.0306513
- Mitra, S., Nandi, M., Mahato, S., Lahiri, D. (2024). Changes in biomarkers of lipid in juvenile idiopathic arthritis and its association with various disease parameters: a 6-month follow-up study. International Journal of Contemporary Pediatrics, 11 (6), 736–743. https://doi.org/10.18203/2349-3291.ijcp20241357
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Olena Onufreiv, Yeva-Emiliia Kulchytska

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.








