Determination of the influence of temperature, concentration of ferric oxides and oxidative conditions of glass boiling on the displacement of the equilibrium of ferric oxides Fе2O3↔FеO
DOI:
https://doi.org/10.15587/2706-5448.2023.283267Keywords:
iron oxides, equilibrium state, redox potential, glass boiling, chemical analysisAbstract
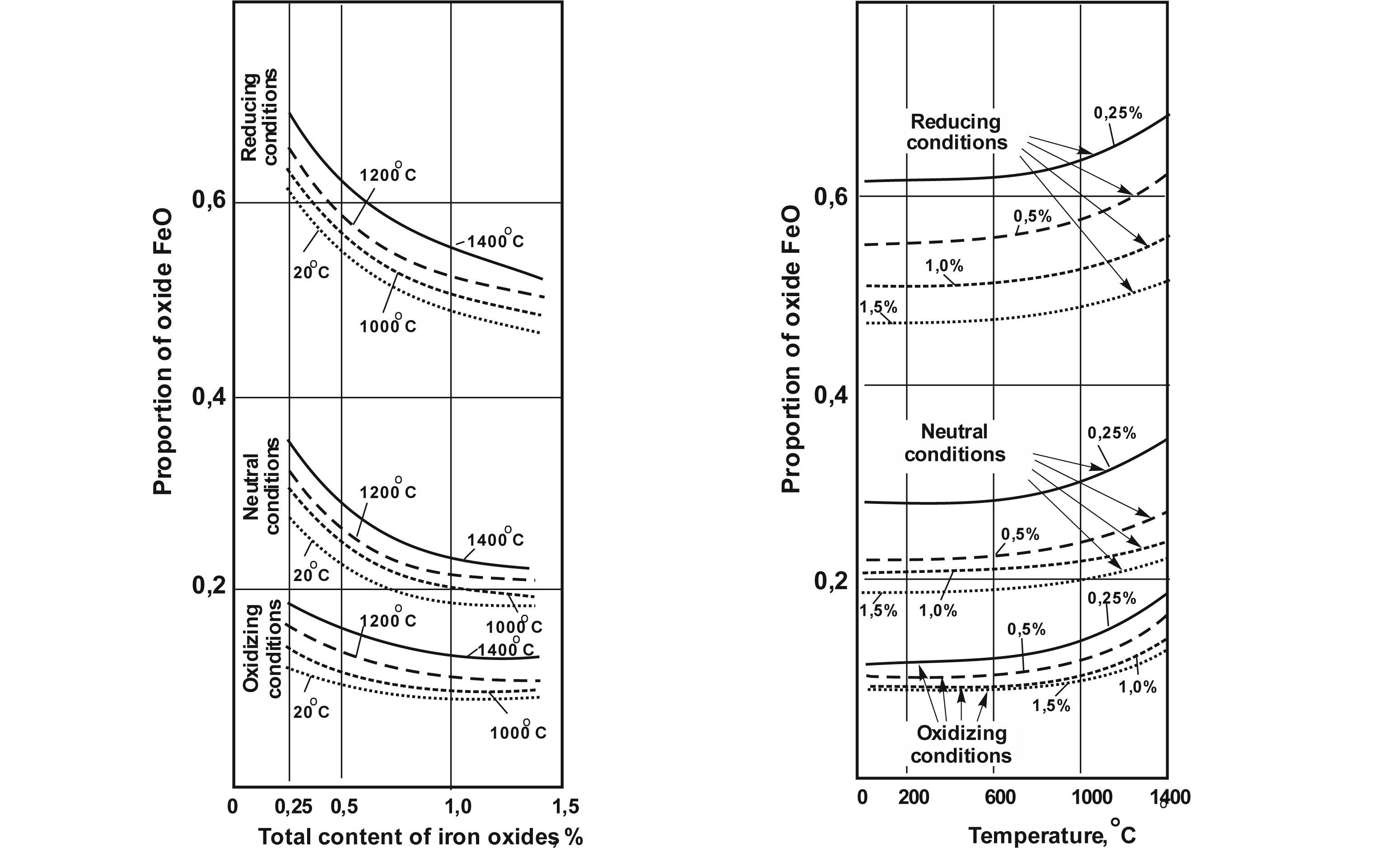
The object of research is the state of equilibrium of ferrum(II) and ferrum(III) oxides in glass melts at temperatures of 1000–1400 °С, welded in oxidizing, neutral and reducing conditions with a content of ferrum oxides up to 1.5 %.
This problem is relevant in the following aspects.
The first aspect of this problem is the unwanted coloring of the glass: FeO colors the glass blue, and Fe2O3 – yellow. The combined presence of ferrum(II) oxide and ferrum(III) oxide determines the gradations of glass shades that fall on the green spectrum.
The second aspect concerns the thermophysics of processes of boiling glasses containing iron oxides. Ferrum(II) oxide causes a strong absorption band of infrared radiation in the region of 1.1 μm. This becomes an obstacle to the volumetric heating of glass in the processes of cooking, forming, and annealing.
The third aspect of the problem concerns the structure of glasses and glass-crystalline materials with an increased content of iron oxides. Iron oxides significantly affect the processes of glass structuring, as ferrum(III) oxide is a typical network former, and ferrum(II) oxide is a typical modifier.
The state of FeO↔Fe2O3 equilibrium in glass is significantly influenced by the glass cooking environment, the total amount of iron oxides, and the temperature of the melt. The glass brewing environment has the greatest influence on the balance of iron oxides in the glass. The share of FeO oxide in the total amount of iron oxides (FeO+Fe2O3) increases sharply when moving from an oxidizing medium to a neutral one and then to a reducing one. During thermostating at a temperature of 1400 °С, the proportion of FeO in the glass increases by 1.4–1.7 times during cooking in an oxidizing environment, by 1.2–1.3 times in a neutral environment, and by approximately 1.1 times in a reducing environment. At the same time, this growth is more noticeable in glasses with a lower iron content.
Thus, the equilibrium state of FeO↔Fe2O3 in glass significantly affects the technological and operational properties of silicate melts and the final glass. The ratio of formed oxides of trivalent and divalent ferrum was studied by chemical (titrometric) analysis.
The research results can be used in practice to develop the composition of glasses with an increased content of iron oxides.
References
- Plemyannikov, M., Zhdaniuk, N. (2020). Study of the possibility of recycling waste of metallurgical products for receipt of glass crystal. Norwegian Journal of Development of the International Science, 42-1, 51–58.
- Vercamer, V. (2016). Spectroscopic and Structural Properties of Iron in Silicate Glasses. Paris, 251.
- Falkovskaia, T. I. (1989). Pogloshchatelnaia sposobnost stekol, soderzhashchikh oksidy elementov perekhodnoi valentnosti v oblasti temperatur 290–1400. Kyiv.
- Calas, G., Petiau, J. (1983). Coordination of iron in oxide glasses through high-resolution K-edge spectra: Information from the pre-edge. Solid State Communications, 48 (7), 625–629. doi: https://doi.org/10.1016/0038-1098(83)90530-6
- Tasheva, T., Harizanova, R., Mihailova, I., Cherkezova‐Zheleva, Z., Paneva, D., Nedkova, M., Rüssel, C. (2023). Structure and redox ratio of soda‐lime‐silica glasses with high iron oxide concentrations. International Journal of Applied Glass Science, 14 (3), 445–454. doi: https://doi.org/10.1111/ijag.16626
- Alderman, O. L. G., Lazareva, L., Wilding, M. C., Benmore, C. J., Heald, S. M., Johnson, C. E. et al. (2017). Local structural variation with oxygen fugacity in Fe2SiO4+fayalitic iron silicate melts. Geochimica et Cosmochimica Acta, 203, 15–36. doi: https://doi.org/10.1016/j.gca.2016.12.038
- Peys, A., White, C. E., Olds, D., Rahier, H., Blanpain, B., Pontikes, Y. (2018). Molecular structure of CaO–FeOx–SiO2 glassy slags and resultant inorganic polymer binders. Journal of the American Ceramic Society, 101 (12), 5846–5857. doi: https://doi.org/10.1111/jace.15880
- Wisniewski, W., Harizanova, R., Völksch, G., Rüssel, C. (2011). Crystallisation of iron containing glass–ceramics and the transformation of hematite to magnetite. CrystEngComm, 13 (12), 4025–4031. doi: https://doi.org/10.1039/c0ce00629g
- Chevrel, M. O., Giordano, D., Potuzak, M., Courtial, P., Dingwell, D. B. (2013). Physical properties of CaAl2Si2O8–CaMgSi2O6–FeO–Fe2O3 melts: Analogues for extra-terrestrial basalt. Chemical Geology, 346, 93–105. doi: https://doi.org/10.1016/j.chemgeo.2012.09.004
- Plemiannikov, M. M., Zhdaniuk, N. V. (2021). Ferrosilicate glass ceramics based on wastes from ore concentration. Voprosy Khimii i Khimicheskoi Tekhnologii, 2, 95–103. doi: https://doi.org/10.32434/0321-4095-2021-135-2-95-103
- Kress, V. C., Carmichael, I. S. E. (1991). The compressibility of silicate liquids containing Fe2O3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contributions to Mineralogy and Petrology, 108 (1-2), 82–92. doi: https://doi.org/10.1007/bf00307328
- Di Genova, D., Hess, K.-U., Chevrel, M. O., Dingwell, D. B. (2016). Models for the estimation of Fe3+/Fetotratio in terrestrial and extraterrestrial alkali- and iron-rich silicate glasses using Raman spectroscopyk. American Mineralogist, 101 (4), 943–952. doi: https://doi.org/10.2138/am-2016-5534ccbyncnd
- Plemiannikov, M. M., Krupa, A. A. (2000). Khimiia ta teplofizyka skla. Kyiv: NTUU «KPI», 560.
- Hughes, E. C., Buse, B., Kearns, S. L., Brooker, R. A., Di Genova, D., Kilgour, G., Mader, H. M., Blundy, J. D. (2020). The microanalysis of iron and sulphur oxidation states in silicate glass – Understanding the effects of beam damage. IOP Conference Series: Materials Science and Engineering, 891 (1), 012014. doi: https://doi.org/10.1088/1757-899x/891/1/012014
- Mysen, B., Virgo, D., Sieferd, F. (1984). Redox equilibria of iron in alkaline earth silicate melts: relationships between melt structure, oxygen fugacity, temperature and properties of iron-bearing silicate liquids. American Mineralogist, 69, 834–847.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Mykola Plemyannikov, Nataliіa Zhdanіuk

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.







