Research on the phenolic profile, antiradical and anti-inflammatory activity of a thick hydroalcoholic feverfew (Tanacetum parthenium L.) herb extract
DOI:
https://doi.org/10.15587/2519-4852.2022.266400Keywords:
Tanacetum parthenium, extract, phenolic profile, hydroxycinnamic acids, antiradical activity, anti-inflammatory activityAbstract
The aim – to study the phenolic complex of a thick hydroalcoholic extract of the feverfew (Tanacetum parthenium (L.) herb (FTHAE), its antiradical activity and anti-inflammatory properties in a model of carrageenan and histamine oedema.
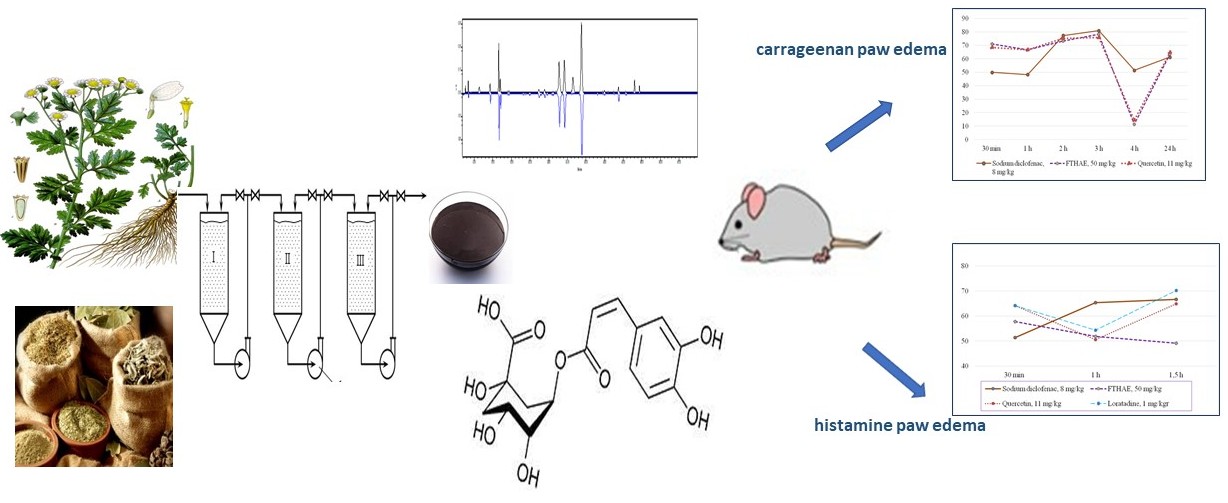
Materials and methods. The studied extract was obtained from the Tanacetum parthenium herb, collected in Sumy and Poltava regions of Ukraine during the period of mass flowering (June-August): degree of grinding of raw materials 2.0-3.0 mm, extraction temperature - 25 °C, extractant – 70 % ethanol, raw material/extractant ratio – 1:12, infusion time – 12 hours, multiplicity of extractions – 3. HPLC and spectrophotometric methods were used to determine the composition and amount of phenolic compounds of FTHAE. HPLC analysis was performed using a “Waters e2695 Alliance system” (Waters, Milford, MA, USA) with a photodiode array detector “Waters 2998” according to the HPLC–PDA method for phenolic compounds. The scavenging of ABTSA radical cation evaluated the radical scavenging activity. In addition, the anti-inflammatory properties of FTHAE were studied on carrageenan and histamine paw oedema in rats. Anti-inflammatory activity (AIA) was evaluated as the ability to reduce oedema compared to the control pathology. FTHAE was used at a dose of 50 mg/kg.
The results. The content of the sum of hydroxycinnamic acids in the obtained extract was determined by spectrophotometry, which was 13.92±0.02 % and the content of the sum of flavonoids – 5.16±0.03 %. The content of 12 compounds with a total amount of 72432.09µg/g was identified and determined by HPLC. The dominant compounds were hydroxycinnamic acids, namely 3,4-dicaffeoylquinic, 4,5-dicaffeoylquinic and сhlorogenic acids. The antiradical activity of the extract was 620.19±4.53µmol/g. On the model of carrageenan oedema, the maximum effect of oedema suppression was 71.0-73.2 %. In the model of histamine oedema, the anti-inflammatory effect of the extract was 57.8; 51.8; and 49.1 % for 30 minutes, 1 and 1.5 hours of oedema, respectively. The severity of the anti-inflammatory activity of the extract during the first hour is not inferior to the diclofenac sodium, quercetin and loratadine.
Conclusions. Due to the HPLC method, 12 compounds were determined to cause antiradical activity, among which chlorogenic acid and rutin were identified.
The studied extract has a pronounced anti-inflammatory effect, which is due to the antiradical properties of the extract and its inhibitory effect on inflammatory mediators
References
- Byts, Yu. V., Butenko, G. M, Gozhenko, A. I. (2015). Pathophysiology. Medicine, 744.
- Scrivo, R., Vasile, M., Bartosiewicz, I., Valesini, G. (2011). Inflammation as “common soil” of the multifactorial diseases. Autoimmunity Reviews, 10 (7), 369–374. https://doi.org/10.1016/j.autrev.2010.12.006
- Botting, R. M., Botting, J. H. (2000). Pathogenesis and Mechanisms of Inflammation and Pain. Clinical Drug Investigation, 19 (Supplement 2), 1–7. https://doi.org/10.2165/00044011-200019002-00001
- Liu, C. H., Abrams, N. D., Carrick, D. M., Chander, P., Dwyer, J., Hamlet, M. R. J. et al. (2017). Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. Nature Immunology, 18 (11), 1175–1180. https://doi.org/10.1038/ni.3828
- Pereira-Leite, C., Nunes, C., Jamal, S. K., Cuccovia, I. M., Reis, S. (2016). Nonsteroidal Anti-Inflammatory Therapy: A Journey Toward Safety. Medicinal Research Reviews, 37 (4), 802–859. https://doi.org/10.1002/med.21424
- Sandoval, A. C., Fernandes, D. R., Silva, E. A. da, Terra Júnior, A. T. (2017). O uso indiscriminado dos Anti-Inflamatórios Não Esteroidais (AINES). Revista Científica FAEMA, 8 (2), 165–176. https://doi.org/10.31072/rcf.v8i2.589
- Sostres, C., Lanas, Á. (2016). Appropriate prescription, adherence and safety of non-steroidal anti-inflammatory drugs. Medicina Clínica, 146 (6), 267–272. https://doi.org/10.1016/j.medcle.2016.05.006
- Onigbinde A.T., M’Kumbuzi V., Olaogun M. O., Oluwafisayo, A. J., Mlenzana, N. B., Shamila, M. et al. (2014). Side Effects of Non-Steroidal Anti-Inflammatory Drugs: The Experience of Patients with Musculoskeletal Disorders. American Journal of Health Research, 2 (4), 106–112. https://doi.org/10.11648/j.ajhr.20140204.11
- Harirforoosh, S., Asghar, W., Jamali, F. (2014). Adverse Effects of Nonsteroidal Antiinflammatory Drugs: An Update of Gastrointestinal, Cardiovascular and Renal Complications. Journal of Pharmacy & Pharmaceutical Sciences, 16 (5), 821–847. https://doi.org/10.18433/j3vw2f
- Kim, K.-H., Seo, H.-J., Abdi, S., Huh, B. (2020). All about pain pharmacology: what pain physicians should know. The Korean Journal of Pain, 33 (2), 108–120. https://doi.org/10.3344/kjp.2020.33.2.108
- Maione, F., Russo, R., Khan, H., Mascolo, N. (2015). Medicinal plants with anti-inflammatory activities. Natural Product Research, 30 (12), 1343–1352. https://doi.org/10.1080/14786419.2015.1062761
- Nunes, C. dos R., Barreto Arantes, M., Menezes de Faria Pereira, S., Leandro da Cruz, L., de Souza Passos, M. et al. (2020). Plants as Sources of Anti-Inflammatory Agents. Molecules, 25 (16), 3726. https://doi.org/10.3390/molecules25163726
- Li, Y., Kong, D., Fu, Y., Sussman, M. R., Wu, H. (2020). The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiology and Biochemistry, 148, 80–89. https://doi.org/10.1016/j.plaphy.2020.01.006
- Zaynab, M., Fatima, M., Abbas, S., Sharif, Y., Umair, M., Zafar, M. H., Bahadar, K. (2018). Role of secondary metabolites in plant defense against pathogens. Microbial Pathogenesis, 124, 198–202. https://doi.org/10.1016/j.micpath.2018.08.034
- Aghmiuni, A. I., Khiavi, A. A. (2017). Medicinal Plants to Calm and Treat Psoriasis Disease. Aromatic and Medicinal Plants – Back to Nature, 28. https://doi.org/10.5772/67062
- Pareek, A., Suthar, M., Rathore, G., Bansal, V. (2011). Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacognosy Reviews, 5 (9), 103–110. https://doi.org/10.4103/0973-7847.79105
- di Giacomo, V., Ferrante, C., Ronci, M., Cataldi, A., Di Valerio, V., Rapino, M. et al. (2019). Multiple pharmacological and toxicological investigations on Tanacetum parthenium and Salix alba extracts: Focus on potential application as anti-migraine agents. Food and Chemical Toxicology, 133, 110783. https://doi.org/10.1016/j.fct.2019.110783
- Hordiei K. R., Gontova T. M. (2020). Study on the composition of fatty and organic acids of the feverfew herb (Tanacetum Parthenium (L.) Schultz Bip.). Farmatsevtychnyi Zhurnal, 5, 61–67. https://doi.org/10.32352/0367-3057.5.20.07
- Liapunov, M., Bezuhla, O., Pidpruzhnykov, Yu. et al. (2011). ST-N MOZU Nastanova 42-3.0:2011.Likarski zasoby. Farmatsevtychna rozrobka (ICHQ8). Kyiv: MOZ Ukrainy. 42.
- Dai, X., Ding, M., Zhang, W., Xuan, Z., Liang, J., Yang, D. et al. (2019). Anti-Inflammatory Effects of Different Elution Fractions of Er-Miao-San on Acute Inflammation Induced by Carrageenan in Rat Paw Tissue. Medical Science Monitor, 25, 7958–7965. https://doi.org/10.12659/msm.916977
- Akhtar, G., Shabbir, A. (2019). Urginea indica attenuated rheumatoid arthritis and inflammatory paw edema in diverse animal models of acute and chronic inflammation. Journal of Ethnopharmacology, 238, 111864. https://doi.org/10.1016/j.jep.2019.111864
- Gontova T. M., Gordei K. R., Mishchenko O. Ya., Kyrychenko, I. V., Kalko, K. O., Kotov A. H. (2020). Pat. No. 140385 UA. Agent with anti-inflammatory. No. u 2019 07427; declareted: 07.04.2019; published: 02.25.2020, Bul. No. 4.
- Fedosov, A. I., Dobrovolnyi, O. O., Shalamay, A. S., Novosel, O. M., Kyslychenko, V. S. (2017). (2017). Comparative analysis of hydroxycinnamic acids of artichoke grown in Ukraine and France. Current issues of pharmaceutical and medical science and practice, 10 (1), 49–53. https://doi.org/10.14739/2409-2932.2017.1.93438
- Krivoruchko, E., Markin, A., Samoilova, Ilina, T., Koshovyi, O. (2018). Research in the chemical composition of the barkof Sorbus aucuparia. Ceska a Slovenska Farmacie, 67 (3), 113–115.
- Zolotaikina, M. Yu., Gontova, T. M., Kotova, E. E., Kotov, A. H., Hubar, S. M. (2016). Development of method for quantitative determination of phenolic compounds in tansy flowers. ScienceRise: Pharmaceutical Science, 1 (1), 34–40. https://doi.org/10.15587/2519-4852.2016.72696
- Raudone, L., Vilkickyte, G., Pitkauskaite, L., Raudonis, R., Vainoriene, R., Motiekaityte, V. (2019). Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules, 24 (5), 844. https://doi.org/10.3390/molecules24050844
- Koshovyi, O., Granica, S., Piwowarski, J. P., Stremoukhov, O., Kostenko, Y., Kravchenko, G. et al. (2021). HighbushBlueberry (Vaccinium corymbosum L.) Leaves Extract and Its Modified Arginine Preparation for the Management of MetabolicSyndrome – Chemical Analysis and Bioactivity in Rat Model. Nutrients, 13 (8), 2870. doi: https://doi.org/10.3390/nu13082870
- Stefanov O. V. (2001). Preclinical studies of drugs (methodological recommendations). Kyiv: VD Avicenna, 528.
- Truhacheva, N. V. (2012). Mathematical statistics in medical-biological researches using the package statistica. Moscow: GEOTAR-Media, 384.
- Hordiei, K., Gontova, T., Kotova, E. et al. (2019). Research on the chemical composition and standartisation of the feverfew thick extract. 10th International Pharmaceutical Conference «Sciences and Practice», Kaunas, 32.
- Marrassini, C., Acevedo, C., Miño, J., Ferraro, G., Gorzalczany, S. (2010). Evaluation of antinociceptive, antinflammatory activities and phytochemical analysis of aerial parts of Urtica urens L. Phytotherapy Research, 24 (12), 1807–1812. https://doi.org/10.1002/ptr.3188
- Emim, J. A. da S., Souccar, C., Castro, M. S. de A., Godinho, R. O., Cezari, M. H. S. et al. (2000). Evidence for activation of the tissue kallikrein-kinin system in nociceptive transmission and inflammatory responses of mice using a specific enzyme inhibitor. British Journal of Pharmacology, 130 (5), 1099–1107. Portico. https://doi.org/10.1038/sj.bjp.0703362
- Broering M. F., Nunes R., Faveri R., De Faveri A. [et al.] (2019). Effects of Tithonia diversifolia (Asteraceae) extract on innate inflammatory responses. J Ethnopharmacol., 242, 112041.
- Miyake, S., Higuchi, H., Honda-Wakasugi, Y., Fujimoto, M., Kawai, H., Nagatsuka, H. et al. (2019). Locally injected ivabradine inhibits carrageenan-induced pain and inflammatory responses via hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. PLOS ONE, 14 (5), e0217209. https://doi.org/10.1371/journal.pone.0217209
- Li, X., Li, K., Xie, H., Xie, Y., Li, Y., Zhao, X., Jiang, X., Chen, D. (2018). Antioxidant and Cytoprotective Effects of the Di-O-Caffeoylquinic Acid Family: The Mechanism, Structure–Activity Relationship, and Conformational Effect. Molecules, 23 (1), 222. https://doi.org/10.3390/molecules23010222
- Hwang, S. J., Kim, Y.-W., Park, Y., Lee, H.-J., Kim, K.-W. (2013). Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation Research, 63 (1), 81–90. https://doi.org/10.1007/s00011-013-0674-4
- Yun, N., Kang, J.-W., Lee, S.-M. (2012). Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. The Journal of Nutritional Biochemistry, 23 (10), 1249–1255. https://doi.org/10.1016/j.jnutbio.2011.06.018
- Benassi-Zanqueta, É., Marques, C. F., Valone, L. M., Pellegrini, B. L., Bauermeister, A., Ferreira, I. C. P. et al. (2019). Evaluation of anti-HSV-1 activity and toxicity of hydroethanolic extract of Tanacetum parthenium (L.) Sch.Bip. (Asteraceae). Phytomedicine, 55, 249–254. https://doi.org/10.1016/j.phymed.2018.06.040
- Chiang, L. C., Chiang, W., Chang, M. Y., Ng, L. T., Lin, C. C. (2002). Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Research, 55 (1), 53–62. https://doi.org/10.1016/s0166-3542(02)00007-4
- Fa, Z., Jianyun, Z., Yiqun, S., Ken, K. (2019). Identification of antioxidative ingredients from feverfew (Tanacetum parthenium) extract substantially free of parthenolide and other alpha-unsaturated gamma-lactones. Open Journal of Analytical and Bioanalytical Chemistry, 3 (1), 076–082. https://doi.org/10.17352/ojabc.000015
- Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., Bitto, A. (2017). Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity, 2017, 1–13. https://doi.org/10.1155/2017/8416763
- Tajner-Czopek, A., Gertchen, M., Rytel, E., Kita, A., Kucharska, A. Z., Sokół-Łętowska, A. (2020). Study of Antioxidant Activity of Some Medicinal Plants Having High Content of Caffeic Acid Derivatives. Antioxidants, 9 (5), 412. https://doi.org/10.3390/antiox9050412
- Miao, M., Xiang, L. (2020). Pharmacological action and potential targets of chlorogenic acid. Advances in Pharmacology, 87, 71–88. https://doi.org/10.1016/bs.apha.2019.12.002
- Xu, J.-G., Hu, Q.-P., Liu, Y. (2012). Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. Journal of Agricultural and Food Chemistry, 60 (46), 11625–11630. https://doi.org/10.1021/jf303771s
- Enogieru, A. B., Haylett, W., Hiss, D. C., Bardien, S., Ekpo, O. E. (2018). Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxidative Medicine and Cellular Longevity, 2018, 1–17. https://doi.org/10.1155/2018/6241017
- Li, Y., Wang, P., Xiao, W., Zhao, L., Wang, Z., Yu, L. (2013). Screening and Analyzing the Potential Bioactive Components from Reduning Injection, Using Macrophage Cell Extraction and Ultra-High Performance Liquid Chromatography Coupled with Mass Spectrometry. The American Journal of Chinese Medicine, 41 (1), 221–229. https://doi.org/10.1142/s0192415x1350016x
- Gao, X., Zhang, S., Wang, L., Yu, L., Zhao, X., Ni, H. et al. (2020). Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) Against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules, 25 (6), 1385. https://doi.org/10.3390/molecules25061385
- Mishchenko, O., Kyrychenko, I., Koshova, O. (2021). Study of certain mechanisms of anti-inflammatory effect of Tanacetum parthenium extract on adjuvant arthritis model in rats. Pharmacologyonline, 3, 367–375.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Oksana Mishchenko, Inna Kyrychenko, Tetiana Gontova, Kateryna Kalko, Karyna Hordiei

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







