Modulation of mesenchymal stromal cells properties by the microenvironment in 3D culture
DOI:
https://doi.org/10.15587/2519-8025.2023.288082Keywords:
mesenchymal stromal cells, three-dimensional cultivation, blood plasma hydrogel, alginate microspheres, macroporous scaffoldsAbstract
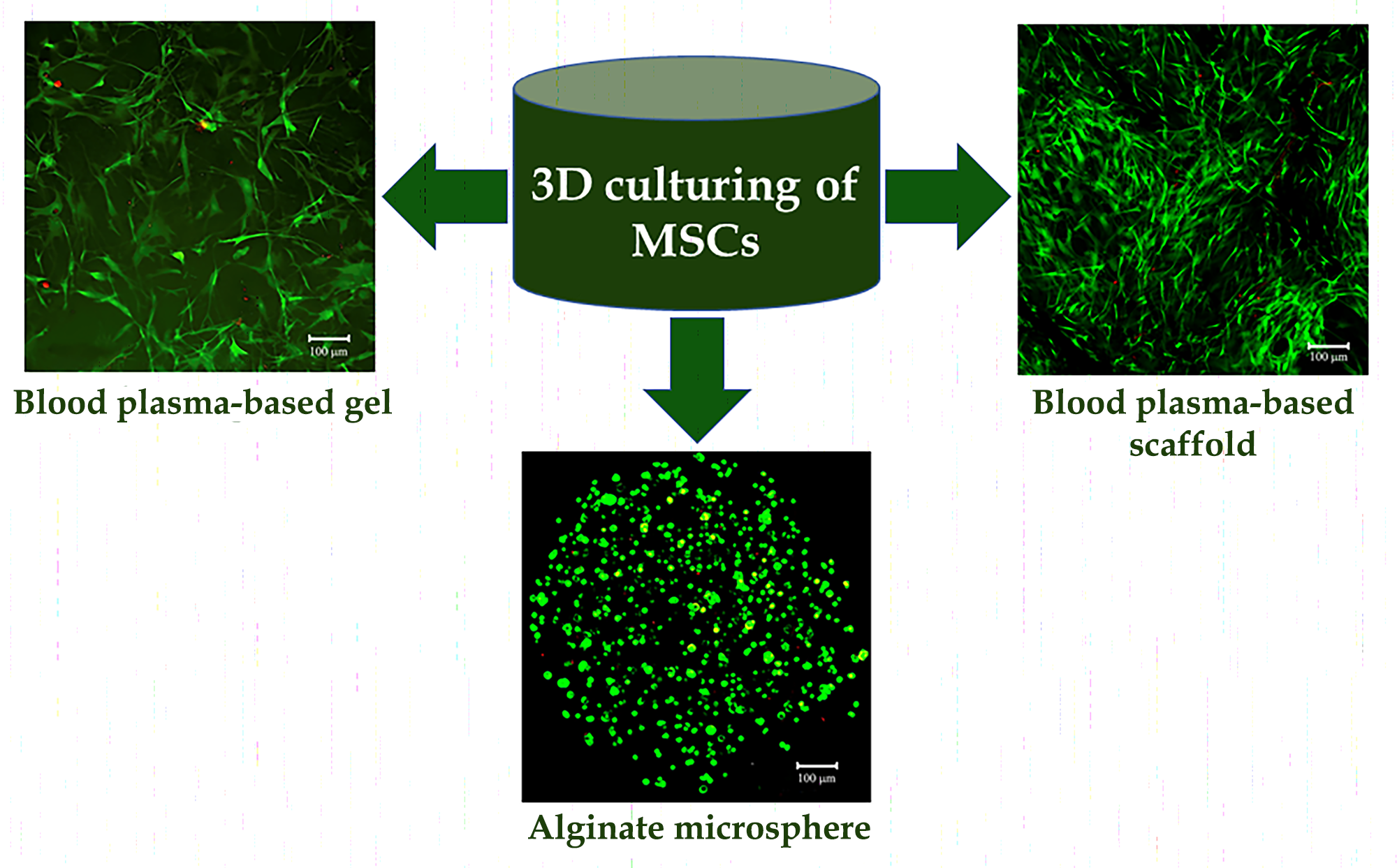
The aim of the research was to compare the shape, viability, metabolic and proliferative activity of mesenchymal stromal cells (MSCs) during cultivation in hydrogels and macroporous scaffolds.
Materials and methods. Human adipose tissue MSCs were isolated from lipoaspirates of healthy adult donors after obtaining informed consent. Hydrogels were obtained from platelet-poor human blood plasma and alginate polymer, cross-linked with calcium ions in microspheres. Macroporous scaffolds were prepared from plasma by the cryotropic gelation method. Morphology and viability of cells within carriers were assessed using vital dyes. Metabolic and proliferative activity of MSCs was studied by the Alamar Blue test on the 1st, 3rd and 7th day of 3D culturing.
Results. Three-dimensional blood plasma scaffolds had a branched pore structure with a size sufficient for cell proliferation and migration. When plasma proteins were cross-linked with L-cysteine, almost all MSCs were viable, attached to the pore surface, spread and proliferated, filling carrier cavities. In plasma hydrogels, MSCs occupied spaces and acquired a fibroblast-like morphology, maintaining viability. In alginate microspheres, MSCs were uniform distributed throughout the gel volume, kept their spherical shape, but had high viability. The highest metabolic activity of MSCs was observed in macroporous scaffolds, the lowest one in alginate microspheres. During cultivation, the activity of cells in macroporous scaffolds and plasma hydrogels increased significantly, which indirectly indicated the proliferation processes.
Conclusions. Properties of MSCs during 3D cultivation significantly depend on the microenvironment: in blood plasma carriers, cells acquire a fibroblast-like morphology and proliferate, while in alginate microspheres, they remain spherical and do not proliferate.
Supporting Agency
- National Research Foundation of Ukraine (project No. 2021.01/0276)
References
- Renesme, L., Pierro, M., Cobey, K. D., Mital, R., Nangle, K., Shorr, R., Lalu, M. M., Thébaud, B. (2022). Definition and Characteristics of Mesenchymal Stromal Cells in Preclinical and Clinical Studies: A Scoping Review. Stem Cells Translational Medicine, 11 (1), 44–54. doi: https://doi.org/10.1093/stcltm/szab009
- Langer, R., Vacanti, J. P. (1993). Tissue Engineering. Science, 260 (5110), 920–926. doi: https://doi.org/10.1126/science.8493529
- Rech, J., Getinger-Panek, A., Gałka, S., Bednarek, I. (2022). Origin and Composition of Exosomes as Crucial Factors in Designing Drug Delivery Systems. Applied Sciences, 12 (23), 12259. doi: https://doi.org/10.3390/app122312259
- Jensen, C., Teng, Y. (2020). Is It Time to Start Transitioning From 2D to 3D Cell Culture? Frontiers in Molecular Biosciences, 7. doi: https://doi.org/10.3389/fmolb.2020.00033
- Tan, L., Liu, X., Dou, H., Hou, Y. (2022). Characteristics and regulation of mesenchymal stem cell plasticity by the microenvironment – specific factors involved in the regulation of MSC plasticity. Genes & Diseases, 9 (2), 296–309. doi: https://doi.org/10.1016/j.gendis.2020.10.006
- Dhamecha, D., Movsas, R., Sano, U., Menon, J. U. (2019). Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. International Journal of Pharmaceutics, 569, 118627. doi: https://doi.org/10.1016/j.ijpharm.2019.118627
- Pravdyuk, A. I., Petrenko, Y. A., Fuller, B. J., Petrenko, A. Y. (2013). Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology, 66 (3), 215–222. doi: https://doi.org/10.1016/j.cryobiol.2013.02.002
- Tarusin, D., Mazur, S., Volkova, N., Petrenko, Yu., Zaikov, V., Petrenko, A. (2016). Encapsulation of mesenchymal stromal cells in alginate microspheres. Biotechnologia Acta, 9 (4), 58–66. doi: https://doi.org/10.15407/biotech9.04.058
- Petrenko, Yu. A., Ivanov, R. V., Petrenko, A. Yu., Lozinsky, V. I. (2011). Coupling of gelatin to inner surfaces of pore walls in spongy alginate-based scaffolds facilitates the adhesion, growth and differentiation of human bone marrow mesenchymal stromal cells. Journal of Materials Science: Materials in Medicine, 22 (6), 1529–1540. doi: https://doi.org/10.1007/s10856-011-4323-6
- Elowsson, L., Kirsebom, H., Carmignac, V., Mattiasson, B., Durbeej, M. (2013). Evaluation of macroporous blood and plasma scaffolds for skeletal muscle tissue engineering. Biomaterials Science, 1 (4), 402–410. doi: https://doi.org/10.1039/c2bm00054g
- Rogulska, O., Petrenko, Y., Petrenko, A. (2016). DMSO-free cryopreservation of adipose-derived mesenchymal stromal cells: expansion medium affects post-thaw survival. Cytotechnology, 69 (2), 265–276. doi: https://doi.org/10.1007/s10616-016-0055-2
- Zuk, P. A., Zhu, M., Ashjian, P., De Ugarte, D. A., Huang, J. I., Mizuno, H. et al. (2002). Human Adipose Tissue Is a Source of Multipotent Stem Cells. Molecular Biology of the Cell, 13 (12), 4279–4295. doi: https://doi.org/10.1091/mbc.e02-02-0105
- Rogulska, O. Y., Trufanova, N. A., Petrenko, Y. A., Repin, N. V., Grischuk, V. P., Ashukina, N. O. et al. (2021). Generation of bone grafts using cryopreserved mesenchymal stromal cells and macroporous collagen‐nanohydroxyapatite cryogels. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 110 (2), 489–499. doi: https://doi.org/10.1002/jbm.b.34927
- Dhurat, R., Sukesh, M. (2014). Principles and methods of preparation of platelet-rich plasma: A review and author′s perspective. Journal of Cutaneous and Aesthetic Surgery, 7 (4), 189–197. doi: https://doi.org/10.4103/0974-2077.150734
- Lozinsky, V. I., Galaev, I. Yu., Plieva, F. M., Savina, I. N., Jungvid, H., Mattiasson, B. (2003). Polymeric cryogels as promising materials of biotechnological interest. Trends in Biotechnology, 21 (10), 445–451. doi: https://doi.org/10.1016/j.tibtech.2003.08.002
- Dankberg, F., Persidsky, M. D. (1976). A test of granulocyte membrane integrity and phagocytic function. Cryobiology, 13 (4), 430–432. doi: https://doi.org/10.1016/0011-2240(76)90098-5
- Petrenko, Yu. A., Gorokhova, N. A., Tkachova, E. N., Petrenko, A. Yu. (2005). The reduction of Alamar Blue by peripheral blood lymphocytes and isolated mitochondria. The Ukrainian Biochemical Journal, 77 (5), 100–105.
- Discher, D. E., Mooney, D. J., Zandstra, P. W. (2009). Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science, 324 (5935), 1673–1677. doi: https://doi.org/10.1126/science.1171643
- de Vos, P., Faas, M. M., Strand, B., Calafiore, R. (2006). Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials, 27 (32), 5603–5617. doi: https://doi.org/10.1016/j.biomaterials.2006.07.010
- Kumar, V. B., Tiwari, O. S., Finkelstein-Zuta, G., Rencus-Lazar, S., Gazit, E. (2023). Design of Functional RGD Peptide-Based Biomaterials for Tissue Engineering. Pharmaceutics, 15 (2), 345. doi: https://doi.org/10.3390/pharmaceutics15020345
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Oleksandr Petrenko, Olena Rogulska, Natalia Trufanova, Oleh Trufanov, Oleksandra Hubenia, Olena Revenko, Daria Cherkashina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







