Evaluation of antimicrobial activity of biomaterials based on alginate and decametoxin against Staphylococus aureus and Escherichia coli
DOI:
https://doi.org/10.15587/2519-8025.2023.298594Keywords:
antimicrobial biomaterials, S.aureus, E.coli, antiseptics, decamethoxin, calcium alginate, antibiotic resistanceAbstract
The aim was to study the antimicrobial activity of new biomaterials based on decamethoxine and commercially available wound dressings against reference and clinical strains of S.aureus and E.coli.
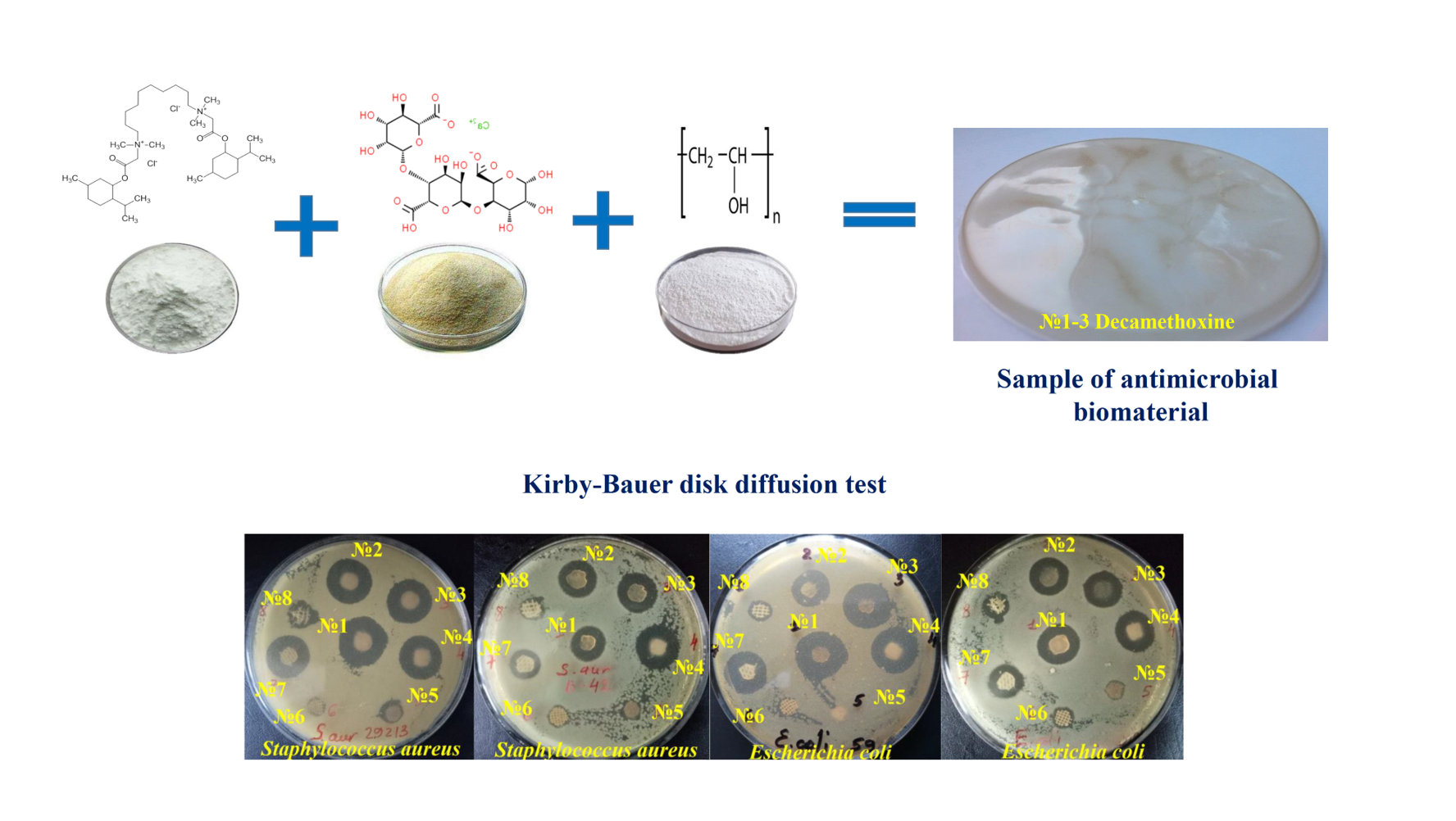
Materials and methods. Developed biomaterials with 0.05 % decamethoxine (DCM No. 1-3) and wound dressings containing antiseptics Suprasorb®, SILVERCEL®, Urgotul SSD®, GUANPOLISEPT®, Bétadine® were used for the study. Antimicrobial properties were studied by zone of inhibition (ZOI) testing using the Kirby-Bauer method.
Results. In relation to S.aureus ATCC 25923, a significantly higher antimicrobial activity of biomaterials with DCM compared to silver- and iodine-containing wound dressings was found to be 1.97-2.11 (p <0.001) and 1.73-1.86 times (p <0.001), respectively. Similar activity against S.aureus ATCC 25923 was possessed by all three samples with DCM (ZOI - from 21.98±0.18 to 23.58±0.26 mm) and Suprasorb® (19.31±0.17 mm), Guanpolisept® (19.13±0.12 mm). Such a tendency was also found in relation to clinical strains of staphylococci. A high level of activity against E.coli ATCC 25922 was shown by biomaterials No. 1-3 DCM (ZOI - from 19.01±0.33 to 21.54±0.23 mm), Guanpolisept® (18.74±0.12 mm) and Suprasorb® (18.43±0.13 mm). Clinical strains of E.coli showed greater tolerance to antimicrobial biomaterials: the difference in mean values between the ZOI of the reference and ZOI of clinical strains of E.coli was significant for all biomaterials (p <0.001). The most effective were biomaterials with DCM No. 1-3 (ZOI - from 15.58±0.25 to 16.41±0.16 mm), as well as Suprasorb® (15.82±0.31 mm).
Conclusions. Biomaterials based on decamethoxine No. 1, No. 2, No. 3, Suprasorb®, Guanpolisept®, and Bétadine® have the highest antistaphylococcal activity. Biomaterials with decamethoxin No.1-3, Suprasorb® and Guanpolisept® show the strongest effect on reference and clinical strains of E.coli
References
- Guiomar, A. J., Urbano, A. M. (2022). Polyhexanide-Releasing Membranes for Antimicrobial Wound Dressings: A Critical Review. Membranes, 12 (12), 1281. doi: https://doi.org/10.3390/membranes12121281
- Liang, Y., Liang, Y., Zhang, H., Guo, B. (2022). Antibacterial biomaterials for skin wound dressing. Asian Journal of Pharmaceutical Sciences, 17 (3), 353–384. doi: https://doi.org/10.1016/j.ajps.2022.01.001
- Boateng, J., Catanzano, O. (2015). Advanced Therapeutic Dressings for Effective Wound Healing – A Review. Journal of Pharmaceutical Sciences, 104 (11), 3653–3680. doi: https://doi.org/10.1002/jps.24610
- Norouzi, M., Boroujeni, S. M., Omidvarkordshouli, N., Soleimani, M. (2015). Advances in Skin Regeneration: Application of Electrospun Scaffolds. Advanced Healthcare Materials, 4 (8), 1114–1133. doi: https://doi.org/10.1002/adhm.201500001
- Pahlevanzadeh, F., Setayeshmehr, M., Bakhsheshi-Rad, H. R., Emadi, R., Kharaziha, M., Poursamar, S. A., Ismail, A. F., Sharif, S., Chen, X., Berto, F. (2022). A Review on Antibacterial Biomaterials in Biomedical Applications: From Materials Perspective to Bioinks Design. Polymers, 14 (11), 2238. doi: https://doi.org/10.3390/polym14112238
- Sam, S., Joseph, B., Thomas, S. (2023). Exploring the antimicrobial features of biomaterials for biomedical applications. Results in Engineering, 17, 100979. doi: https://doi.org/10.1016/j.rineng.2023.100979
- Yu, R., Zhang, H., Guo, B. (2021). Conductive Biomaterials as Bioactive Wound Dressing for Wound Healing and Skin Tissue Engineering. Nano-Micro Letters, 14 (1). doi: https://doi.org/10.1007/s40820-021-00751-y
- Dodero, A., Scarfi, S., Pozzolini, M., Vicini, S., Alloisio, M., Castellano, M. (2019). Alginate-Based Electrospun Membranes Containing ZnO Nanoparticles as Potential Wound Healing Patches: Biological, Mechanical, and Physicochemical Characterization. ACS Applied Materials & Interfaces, 12 (3), 3371–3381. doi: https://doi.org/10.1021/acsami.9b17597
- Da Silva, J., Leal, E. C., Carvalho, E., Silva, E. A. (2023). Innovative Functional Biomaterials as Therapeutic Wound Dressings for Chronic Diabetic Foot Ulcers. International Journal of Molecular Sciences, 24 (12), 9900. doi: https://doi.org/10.3390/ijms24129900
- Simões, D., Miguel, S. P., Ribeiro, M. P., Coutinho, P., Mendonça, A. G., Correia, I. J. (2018). Recent advances on antimicrobial wound dressing: A review. European Journal of Pharmaceutics and Biopharmaceutics, 127, 130–141. doi: https://doi.org/10.1016/j.ejpb.2018.02.022
- Falk, N. A. (2019). Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. Journal of Surfactants and Detergents, 22(5), 1119–1127. doi: https://doi.org/10.1002/jsde.12293
- Babalska, Z. Ł., Korbecka-Paczkowska, M., Karpiński, T. M. (2021). Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals, 14 (12), 1253. doi: https://doi.org/10.3390/ph14121253
- Joyce, K., Fabra, G. T., Bozkurt, Y., Pandit, A. (2021). Bioactive potential of natural biomaterials: identification, retention and assessment of biological properties. Signal Transduction and Targeted Therapy, 6 (1). doi: https://doi.org/10.1038/s41392-021-00512-8
- EUCAST disk diffusion test methodology (2015). European committee on antimicrobial susceptibility testing (EUCAST). Available at: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology/ Last accessed: 12.08.2015
- Routine and extended internal quality control for MIC determination and disk diffusion as recommended by EUCAST version 12.0 (2022). European Committee on Antimicrobial Susceptibility Testing; Växjö.
- Matuschek, E., Longshaw, C., Takemura, M., Yamano, Y., Kahlmeter, G. (2022). Cefiderocol: EUCAST criteria for disc diffusion and broth microdilution for antimicrobial susceptibility testing. Journal of Antimicrobial Chemotherapy, 77 (6), 1662–1669. doi: https://doi.org/10.1093/jac/dkac080
- Antimicrobial Susceptibility Testing, EUCAST Disk Diffusion Method, Version 11.0 (2023). The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Available at: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2023_manuals/Manual_v_11.0_EUCAST_Disk_Test_2023.pdf Last accessed: 10.01.2023
- Chambers, H. F., DeLeo, F. R. (2009). Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Reviews Microbiology, 7 (9), 629–641. doi: https://doi.org/10.1038/nrmicro2200
- Ejaz, M., Syed, M. A., Jackson, C. R., Sharif, M., Faryal, R. (2023). Epidemiology of Staphylococcus aureus Non-Susceptible to Vancomycin in South Asia. Antibiotics, 12 (6), 972. doi: https://doi.org/10.3390/antibiotics12060972
- Reich, P. J., Boyle, M. G., Hogan, P. G., Johnson, A. J., Wallace, M. A., Elward, A. M. et al. (2016). Emergence of community-associated methicillin-resistant Staphylococcus aureus strains in the neonatal intensive care unit: an infection prevention and patient safety challenge. Clinical Microbiology and Infection, 22 (7), 645.e1–645.e8. doi: https://doi.org/10.1016/j.cmi.2016.04.013
- Yang, E. S., Tan, J., Eells, S., Rieg, G., Tagudar, G., Miller, L. G. (2010). Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clinical Microbiology and Infection, 16 (5), 425–431. doi: https://doi.org/10.1111/j.1469-0691.2009.02836.x
- Linz, M. S., Mattappallil, A., Finkel, D., Parker, D. (2023). Clinical Impact of Staphylococcus aureus Skin and Soft Tissue Infections. Antibiotics, 12 (3), 557. doi: https://doi.org/10.3390/antibiotics12030557
- Esposito, S., Blasi, F., Curtis, N., Kaplan, S., Lazzarotto, T., Meschiari, M. et al. (2023). New Antibiotics for Staphylococcus aureus Infection: An Update from the World Association of Infectious Diseases and Immunological Disorders (WAidid) and the Italian Society of Anti-Infective Therapy (SITA). Antibiotics, 12 (4), 742. doi: https://doi.org/10.3390/antibiotics12040742
- Upreti, N., Rayamajhee, B., Sherchan, S. P., Choudhari, M. K., Banjara, M. R. (2018). Prevalence of methicillin resistant Staphylococcus aureus, multidrug resistant and extended spectrum β-lactamase producing gram negative bacilli causing wound infections at a tertiary care hospital of Nepal. Antimicrobial Resistance & Infection Control, 7 (1). doi: https://doi.org/10.1186/s13756-018-0408-z
- Tefera, S., Awoke, T., Mekonnen, D. (2021). Methicillin and Vancomycin Resistant Staphylococcus aureus and Associated Factors from Surgical Ward Inpatients at Debre Markos Referral Hospital, Northwest Ethiopia. Infection and Drug Resistance, 14, 3053–3062. doi: https://doi.org/10.2147/idr.s324042
- Braz, V. S., Melchior, K., Moreira, C. G. (2020). Escherichia coli as a Multifaceted Pathogenic and Versatile Bacterium. Frontiers in Cellular and Infection Microbiology, 10. doi: https://doi.org/10.3389/fcimb.2020.548492
- Wilcox, M. H., Dryden, M. (2021). Update on the epidemiology of healthcare-acquired bacterial infections: focus on complicated skin and skin structure infections. Journal of Antimicrobial Chemotherapy, 76 (4), iv2–iv8. doi: https://doi.org/10.1093/jac/dkab350
- Puca, V., Marulli, R. Z., Grande, R., Vitale, I., Niro, A., Molinaro, G. et al. (2021). Microbial Species Isolated from Infected Wounds and Antimicrobial Resistance Analysis: Data Emerging from a Three-Years Retrospective Study. Antibiotics, 10 (10), 1162. doi: https://doi.org/10.3390/antibiotics10101162
- Urase, T., Okazaki, M., Tsutsui, H. (2020). Prevalence of ESBL-producing Escherichia coli and carbapenem-resistant Enterobacteriaceae in treated wastewater: a comparison with nosocomial infection surveillance. Journal of Water and Health, 18 (6), 899–910. doi: https://doi.org/10.2166/wh.2020.014
- Tian, X., Sun, S., Jia, X., Zou, H., Li, S., Zhang, L. (2018). Epidemiology of and risk factors for infection with extended-spectrum β-lactamase-producing carbapenem-resistant Enterobacteriaceae: results of a double case–control study. Infection and Drug Resistance, 11, 1339–1346. doi: https://doi.org/10.2147/idr.s173456
- Kramer, A., Dissemond, J., Kim, S., Willy, C., Mayer, D., Papke, R. et al. (2017). Consensus on Wound Antisepsis: Update 2018. Skin Pharmacology and Physiology, 31 (1), 28–58. doi: https://doi.org/10.1159/000481545
- Yousefian, F., Hesari, R., Jensen, T., Obagi, S., Rgeai, A., Damiani, G. et al. (2023). Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics, 12 (9), 1434. doi: https://doi.org/10.3390/antibiotics12091434
- Nazarchuk, O. (2019). Research of antimicrobial efficacy of modern antiseptic agents based on decamethoxine and povidone-iodine. Perioperaciina Medicina, 2 (1), 6–10. doi: https://doi.org/10.31636/prmd.v2i1.1
- Garcia, L. V., Silva, D., Costa, M. M., Armés, H., Salema-Oom, M., Saramago, B., Serro, A. P. (2023). Antiseptic-Loaded Casein Hydrogels for Wound Dressings. Pharmaceutics, 15 (2), 334. doi: https://doi.org/10.3390/pharmaceutics15020334
- Eberlein, T., Haemmerle, G., Signer, M., Gruber-Moesenbacher, U., Traber, J., Mittlboeck, M., Abel, M., Strohal, R. (2012). Comparison of PHMB-containing dressing and silver dressings in patients with critically colonised or locally infected wounds. Journal of Wound Care, 2 1(1), 12–20. doi: https://doi.org/10.12968/jowc.2012.21.1.12
- Dydak, K., Junka, A., Dydak, A., Brożyna, M., Paleczny, J., Fijalkowski, K. et al. (2021). In Vitro Efficacy of Bacterial Cellulose Dressings Chemisorbed with Antiseptics against Biofilm Formed by Pathogens Isolated from Chronic Wounds. International Journal of Molecular Sciences, 22 (8), 3996. doi: https://doi.org/10.3390/ijms22083996
- Rippon, M. G., Rogers, A. A., Ousey, K. (2023). Polyhexamethylene biguanide and its antimicrobial role in wound healing: a narrative review. Journal of Wound Care, 32 (1), 5–20. doi: https://doi.org/10.12968/jowc.2023.32.1.5
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Oleksandr Nazarchuk, Tetyana Denysko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







