Antimicrobial activity of a new compound of 1,2,4-triazole derivatives against pathogens of poultry bacterial diseases
DOI:
https://doi.org/10.15587/2519-8025.2024.311824Keywords:
1,2,4 triazole derivatives, antibiotic resistance, strains, MIC, MBC, antimicrobial activity, organic synthesis, heterocyclic compounds, bactericidal action, inhibitory actionAbstract
The aim: study of the antimicrobial activity of a new compound of 1,2,4-triazole derivatives against pathogens of poultry bacteriosis.
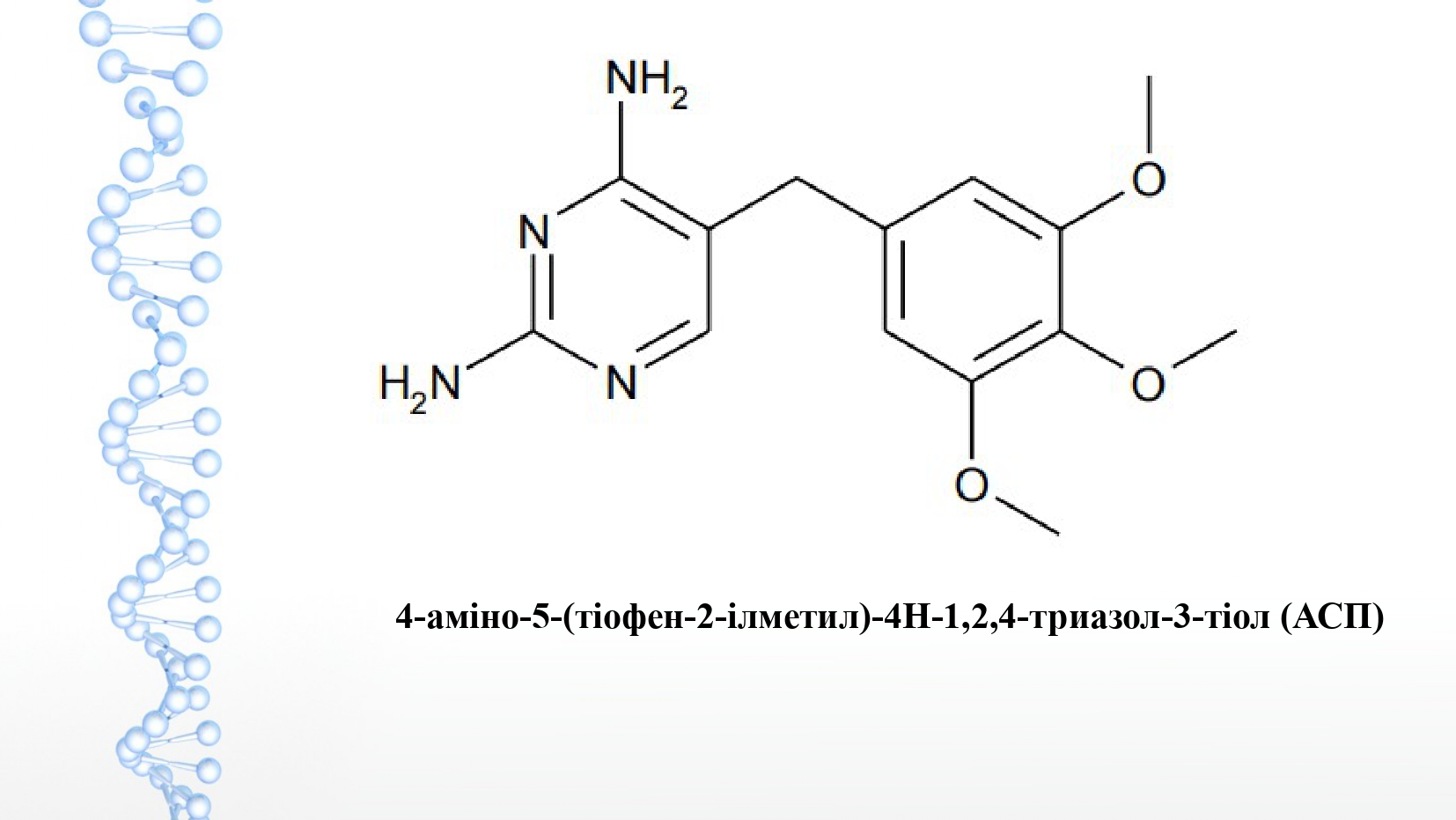
Materials and methods. Research was conducted on the bactericidal activity of a new compound of 1,2,4-triazole derivatives against pathogens of poultry bacteriosis. Dimethylsulfoxide was used to dissolve the ASP compound. In order to study the bactericidal activity of the compounds, reference and field strains of S. typhimurium, E. coli, St. aureus, P. aeruginosa, P. vulgaris, K. pneumoniae, L. monocytogenes, E. faecalis were used. The study of the minimum inhibitory concentration (MIC) was carried out by the method of serial dilution in Muller-Hinton broth, the minimum bactericidal concentration (MBC) - by seeding from transparent tubes on Petri dishes with differential nutrient media.
The results. The inhibitory effect of the ASP compound at a concentration of 62.5 μg/ml against E. coli, L. Monocytogenes, E. faecalis, and at a concentration of 125 μg/ml against S. typhimurium, E. coli, St. aureus, P. aeruginosa, P. vulgaris, K. pneumoniae, L. monocytogenes, E. faecalis was established. The bactericidal effect of the ASP compound was detected at a concentration of 62.5 μg/ml against L. monocytogenes, at a concentration of 125 μg/ml - against E. coli, St. aureus, L. monocytogenes and E. faecalis. The ASP compound at a concentration of 250 μg/ml has the inhibitory and bactericidal effect on all tested reference and field strains of poultry bacteriosis pathogens.
Conclusions. A new synthesized compound of 1,2,4 triazole derivatives of ASP exhibits the inhibitory effect at a concentration of 125 μg/ml - in relation to E. coli, St. aureus, L. monocytogenes and E. faecalis and the bactericidal effect against bacteriosis pathogens S. typhimurium, E. coli, St. aureus, P. aeruginosa, P. vulgaris, K. pneumoniae, L. monocytogenes, E. faecalis at a concentration of 250 μg/ml
References
- Duval, R. E., Grare, M., Demoré, B. (2019). Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules, 24 (17), 3152. https://doi.org/10.3390/molecules24173152
- Palma, E., Tilocca, B., Roncada, P. (2020). Antimicrobial Resistance in Veterinary Medicine: An Overview. International Journal of Molecular Sciences, 21 (6), 1914. https://doi.org/10.3390/ijms21061914
- Holmes, A. H., Moore, L. S. P., Sundsfjord, A., Steinbakk, M., Regmi, S., Karkey, A. et al. (2016). Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet, 387 (10014), 176–187. https://doi.org/10.1016/s0140-6736(15)00473-0
- Strzelecka, M., Świątek, P. (2021). 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals, 14 (3), 224. https://doi.org/10.3390/ph14030224
- Ezelarab, H. A. A., Abbas, S. H., Hassan, H. A., Abuo‐Rahma, G. E. A. (2018). Recent updates of fluoroquinolones as antibacterial agents. Archiv Der Pharmazie, 351 (9). https://doi.org/10.1002/ardp.201800141
- Muthal, N., Ahirwar, J., Ahriwar, D., Masih, P., Mahmdapure, T., Sivakumar, T. (2010). Synthesis, Antimicrobial and Anti-Inflammatory Activity of Some 5-Substituted-3-Pyridine-1, 2, 4-Triazoles. International Journal of PharmTech Research, 2, 2450–2455.
- Singh, R., Pujar, G. V., Purohit, M. N., Chandrashekar, V. M. (2012). Synthesis, in vitro cytotoxicity, and antibacterial studies of new asymmetric bis-1,2,4-triazoles. Medicinal Chemistry Research, 22 (5), 2163–2173. https://doi.org/10.1007/s00044-012-0209-5
- Peng, Z., Wang, G., Zeng, Q.-H., Li, Y., Wu, Y., Liu, H., Wang, J. J., Zhao, Y. (2021). Synthesis, antioxidant and anti-tyrosinase activity of 1,2,4-triazole hydrazones as antibrowning agents. Food Chemistry, 341, 128265. https://doi.org/10.1016/j.foodchem.2020.128265
- Grytsai, O., Valiashko, O., Penco-Campillo, M., Dufies, M., Hagege, A., Demange, L. et al. (2020). Synthesis and biological evaluation of 3-amino-1,2,4-triazole derivatives as potential anticancer compounds. Bioorganic Chemistry, 104, 104271. https://doi.org/10.1016/j.bioorg.2020.104271
- Harkavenko, T. O., Nevolko, O. M., Kozytska, T. H. (2014). Metodychni vkazivky shchodo vyznachennia chutlyvosti mikroorhanizmiv do antybakterialnykh preparativ. Kyiv: DNDILDVSE, 54.
- Cox, J. R., Woodcock, Stephen., Hillier, I. H., Vincent, M. A. (1990). Tautomerism of 1,2,3- and 1,2,4-triazole in the gas phase and in aqueous solution: a combined ab initio quantum mechanics and free energy perturbation study. The Journal of Physical Chemistry, 94 (14), 5499–5501. https://doi.org/10.1021/j100377a016
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Yevheniia Vashchyk, Andriy Safonov, Andriy Zakhariev, Olga Shapovalova, Denys Demianenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







