Botulotoxin as the most typical product of the biotechnology for aesthetic cosmetology and the study of subjective attitude towards its use among the Ukrainian population sample
DOI:
https://doi.org/10.15587/2519-8025.2024.320781Keywords:
Botulinum therapy, attitude towards botulinum therapy, awareness of botulinum toxin, population of UkraineAbstract
The aim of the study was to analyze the effectiveness of botulinum therapy in aesthetic cosmetology and to consider the influence of various factors on the decision to perform the procedure.
Materials and methods. The work used content analysis of literary sources, analytical method, and online survey of females and males of different generations regarding the botulinum therapy procedure.
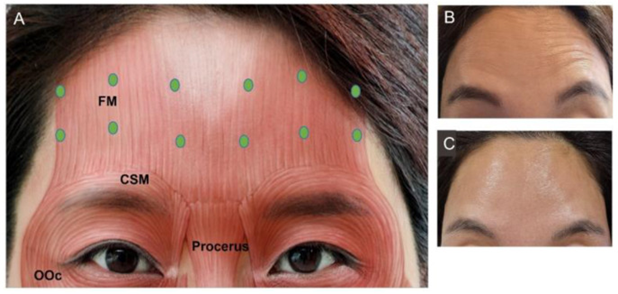
Results and discussion. Botulinum toxin was historically first used to smooth glabellar wrinkles, and then its use was expanded to other areas of the face. There are seven types of botulinum toxin (A, B, C, D, E, F, and G). Experience with the use of botulinum therapy for aesthetic purposes shows that clinical ineffectiveness is extremely rare. Depending on gender, the reaction to procedures with the introduction of botulinum toxin for cosmetic purposes is different. Females are more aware of the botulinum therapy procedure than males. Botulinum toxin therapy is most common among younger adults (25 to 39 years old).
Conclusions. Interest in botulinum toxin therapy increases with age. People, interested in botulinum toxin therapy, are more likely to visit a cosmetologist regularly. Profession affects person’s involvement in the botulinum toxin therapy procedure. The largest proportion of the respondents who have undergone botulinum toxin therapy is observed among the medical and beauty sectors, business and management. The largest number of the respondents who want to try botulinum toxin therapy is among students. This may indicate the openness of the younger generation to experiments and understanding the importance of continuing their youth in the future for as long as possible. The study showed that BMI does not affect respondents’ involvement in the botulinum toxin therapy procedure. The Internet is the main source of information about botulinum toxin therapy for a significant number of the respondents, regardless of their involvement in this procedure. The participants prioritize the result of botulinum toxin therapy, not its cost
References
- Moriarty, K. C. (2003). Botulinum Toxin in Facial Rejuvenation. Mosby, 180. https://doi.org/10.1016/b978-0-7234-3349-1.x5002-1
- Ascher, B., Zakine, B., Kestemont, P., Baspeyras, M., Bougara, A., Santini, J. (2004). A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. Journal of the American Academy of Dermatology, 51 (2), 223–233. https://doi.org/10.1016/j.jaad.2003.11.084
- DeBoulle, K., Glogau, R., Fagien, S., Sommer, B. (2010). Treating glabellar lines with botulinum toxin type A-hemagglutinin complex: A review of the science, the clinical data, and patient satisfaction. Clinical Interventions in Aging, 5, 101–118. https://doi.org/10.2147/cia.s9338
- Carruthers, J. D. A., Carruthers, J. A. (1992). Treatment of Glabellar Frown Lines with C. Botulinum‐A Exotoxin. The Journal of Dermatologic Surgery and Oncology, 18 (1), 17–21. https://doi.org/10.1111/j.1524-4725.1992.tb03295.x
- Truong, D., Dressler, D., Hallett, M. (Eds.) (2009). Manual of Botulinum Toxin Therapy. New York: Cambridge University Press. https://doi.org/10.1017/cbo9780511575761
- Dolly, J. O., Aoki, K. R. (2006). The structure and mode of action of different botulinum toxins. European Journal of Neurology, 13 (s4), 1–9. https://doi.org/10.1111/j.1468-1331.2006.01648.x
- Arsenault, J., Cuijpers, S. A. G., Ferrari, E., Niranjan, D., Rust, A., Leese, C. et al. (2014). Botulinum protease‐cleaved SNARE fragments induce cytotoxicity in neuroblastoma cells. Journal of Neurochemistry, 129 (5), 781–791. https://doi.org/10.1111/jnc.12645
- Pirazzini, M., Rossetto, O., Eleopra, R., Montecucco, C., Witkin, J. M. (2017). Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacological Reviews, 69 (2), 200–235. https://doi.org/10.1124/pr.116.012658
- Wollina, U., Konrad, H., Petersen, S. (2005). Botulinum toxin in dermatology – beyond wrinkles and sweat. Journal of Cosmetic Dermatology, 4 (4), 223–227. https://doi.org/10.1111/j.1473-2165.2005.00195.x
- Glogau, R. G. (2008). Botulinum toxin. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw Hill, 2389–2395.
- Flynn, T. C. (2012). Advances in the use of botulinum neurotoxins in facial esthetics. Journal of Cosmetic Dermatology, 11 (1), 42–50. https://doi.org/10.1111/j.1473-2165.2011.00593.x
- Khawaja, H. A., Hernandez‐Perez, E. (2001). Botox in dermatology. International Journal of Dermatology, 40 (5), 311–317. https://doi.org/10.1046/j.1365-4362.2001.01176.x
- Lowe, N. J. (2010). Minimally Invasive Treatments and Procedures for Ageing Skin. Rook’s Textbook of Dermatology. London: Blackwell Publishing, 80.1–80.14. https://doi.org/10.1002/9781444317633.ch80
- U.S. Food and Drug Administration. Available at: https://www.fda.gov/ Last accessed: 30.04.2024
- Carruthers, J., Carruthers, A. (2007). The evolution of botulinum neurotoxin type A for cosmetic applications. Journal of Cosmetic and Laser Therapy, 9 (3), 186–192. https://doi.org/10.1080/14764170701411470
- Trindade De Almeida, A. R., Secco, L. C., Carruthers, A. (2011). Handling Botulinum Toxins. Dermatologic Surgery, 37 (11), 1553–1565. https://doi.org/10.1111/j.1524-4725.2011.02087.x
- Kranz, G., Haubenberger, D., Voller, B., Posch, M., Schnider, P., Auff, E., Sycha, T. (2008). Respective potencies of Botox® and Dysport® in a human skin model: A randomized, double‐blind study. Movement Disorders, 24 (2), 231–236. https://doi.org/10.1002/mds.22336
- Jost, W. H., Blumel, J., Grafe, S. (2007). Botulinum neurotoxin type A free of complexing proteins (XEOMIN®) in focal dystonia. Drugs, 67 (5), 669–683. https://doi.org/10.2165/00003495-200767050-00003
- Rieder, C. R. M., Schestatsky, P., Socal, M. P., Monte, T. L., Fricke, D., Costa, J., Picon, P. D. (2007). A Double-blind, Randomized, Crossover Study of Prosigne Versus Botox in Patients With Blepharospasm and Hemifacial Spasm. Clinical Neuropharmacology, 30 (1), 39–42. https://doi.org/10.1097/01.wnf.0000236771.77021.3c
- Hunt, T., Clarke, K. (2008). Potency of the botulinum toxin product CNBTX-A significantly exceeds labeled units in standard potency test. Journal of the American Academy of Dermatology, 58 (3), 517–518. https://doi.org/10.1016/j.jaad.2007.11.015
- Spencer, J. M., Gordon, M., Goldberg, D. J. (2002). Botulinum B treatment of the glabellar and frontalis regions: a dose response analysis. Journal of Cosmetic and Laser Therapy, 4 (1), 19–23. https://doi.org/10.1080/14764170260030126
- Jacob, C. I. (2003). Botulinum neurotoxin type B – A rapid wrinkle reducer. Seminars in Cutaneous Medicine and Surgery, 22 (2), 131–135. https://doi.org/10.1053/sder.2003.50009
- Lambros, V. (2008). Models of Facial Aging and Implications for Treatment. Clinics in Plastic Surgery, 35 (3), 319–327. https://doi.org/10.1016/j.cps.2008.02.012
- Fagien, S., Carruthers, J. D. A. (2008). A Comprehensive Review of Patient-Reported Satisfaction with Botulinum Toxin Type A for Aesthetic Procedures. Plastic and Reconstructive Surgery, 122 (6), 1915–1925. https://doi.org/10.1097/prs.0b013e31818dbfe3
- Wieder, J. M., Moy, R. L. (1998). Understanding Botulinum Toxin. Dermatologic Surgery, 24 (11), 1172–1174. https://doi.org/10.1111/j.1524-4725.1998.tb04093.x
- Kwon, K.-H., Shin, K. S., Yeon, S. H., Kwon, D. G. (2019). Application of botulinum toxin in maxillofacial field: part I. Bruxism and square jaw. Maxillofacial Plastic and Reconstructive Surgery, 41 (1). https://doi.org/10.1186/s40902-019-0218-0
- Janes, L. E., Connor, L. M., Moradi, A., Alghoul, M. (2021). Current Use of Cosmetic Toxins to Improve Facial Aesthetics. Plastic & Reconstructive Surgery, 147 (4), 644e–657e. https://doi.org/10.1097/prs.0000000000007762
- Small, R. (2014). Botulinum toxin injection for facial wrinkles. American Family Physician, 90, 168–175.
- Maas, C., Kane, M. A. C., Bucay, V. W., Allen, S., Applebaum, D. J., Baumann, L. et al. (2012). Current Aesthetic Use of AbobotulinumtoxinA in Clinical Practice: An Evidence-Based Consensus Review. Aesthetic Surgery Journal, 32 (1_Supplement), 8S–29S. https://doi.org/10.1177/1090820x12455192
- Sundaram, H., Huang, P.-H., Hsu, N.-J., Huh, C. H., Wu, W. T. L., Wu, Y. et al. (2016). Aesthetic Applications of Botulinum Toxin A in Asians: An International, Multidisciplinary, Pan-Asian Consensus. Plastic and Reconstructive Surgery - Global Open, 4 (12), e872. https://doi.org/10.1097/gox.0000000000000507
- Frevert, J., Ahn, K. Y., Park, M. Y., Sunga, O. (2018). Comparison of botulinum neurotoxin type A formulations in Asia. Clinical, Cosmetic and Investigational Dermatology, 11, 327–331. https://doi.org/10.2147/ccid.s160723
- Jaspers, G. W. C., Pijpe, J., Jansma, J. (2011). The use of botulinum toxin type A in cosmetic facial procedures. International Journal of Oral and Maxillofacial Surgery, 40 (2), 127–133. https://doi.org/10.1016/j.ijom.2010.09.014
- Kim, H.-J., Youn, K.-H., Kim, J.-S., Kim, Y. S., Hong, S. O., Na, J. (2020). US Applications in Botulinum Toxin Injection Procedures. Ultrasonographic Anatomy of the Face and Neck for Minimally Invasive Procedures. Singapore, 215–241. https://doi.org/10.1007/978-981-15-6560-1_8
- Hong, S. O. (2023). Cosmetic Treatment Using Botulinum Toxin in the Oral and Maxillofacial Area: A Narrative Review of Esthetic Techniques. Toxins, 15 (2), 82. https://doi.org/10.3390/toxins15020082
- Kim, H.-J., Seo, K. K., Lee, H.-K., Kim, J. (2016). Clinical Anatomy for Botulinum Toxin Injection. Clinical Anatomy of the Face for Filler and Botulinum Toxin Injection. Singapore, 55–92. https://doi.org/10.1007/978-981-10-0240-3_2
- Kim, H.-J., Youn, K.-H., Kim, J.-S., Kim, Y. S., Hong, S. O., Na, J. (2020). General US Anatomy of the Face and Neck. Ultrasonographic Anatomy of the Face and Neck for Minimally Invasive Procedures. Singapore, 25–73. https://doi.org/10.1007/978-981-15-6560-1_2
- Carruthers, J., Fagien, S., Matarasso, S. L. (2004). Consensus Recommendations on the Use of Botulinum Toxin Type A in Facial Aesthetics. Plastic and Reconstructive Surgery, 114 (Supplement), 1S-22S. https://doi.org/10.1097/01.prs.0000144795.76040.d3
- Gart, M. S., Gutowski, K. A. (2016). Overview of Botulinum Toxins for Aesthetic Uses. Clinics in Plastic Surgery, 43 (3), 459–471. https://doi.org/10.1016/j.cps.2016.03.003
- Hwang, W.-S., Hur, M.-S., Hu, K.-S., Song, W.-C., Koh, K.-S., Baik, H.-S. et al. (2009). Surface Anatomy of the Lip Elevator Muscles for the Treatment of Gummy Smile Using Botulinum Toxin. The Angle Orthodontist, 79 (1), 70–77. https://doi.org/10.2319/091407-437.1
- Rzany, B. (2007). Requirements and Rules. Botulinum Toxin in Aesthetic Medicine, 21–24. https://doi.org/10.1007/978-3-540-34095-9_3
- de Maio, M., Wu, W. T. L., Goodman, G. J., Monheit, G. (2017). Facial Assessment and Injection Guide for Botulinum Toxin and Injectable Hyaluronic Acid Fillers: Focus on the Lower Face. Plastic & Reconstructive Surgery, 140 (3), 393e–404e. https://doi.org/10.1097/prs.0000000000003646
- Keaney, T. C., Alster, T. S. (2013). Botulinum toxin in men: review of relevant anatomy and clinical trial data. Dermatologic Surgery, 39 (10), 1434–1443.
- Schlessinger, J., Dover, J. S., Joseph, J., Monheit, G., Nelson, D. B., Albright, C. D. et al. (2014). Long-Term Safety of AbobotulinumtoxinA for the Treatment of Glabellar Lines: Results From a 36-Month, Multicenter, Open-Label Extension Study. Dermatologic Surgery, 40 (2), 176–183. https://doi.org/10.1111/dsu.12404
- Kundu, N., Kothari, R., Shah, N., Sandhu, S., Tripathy, D. M., Galadari, H. et al. (2022). Efficacy of botulinum toxin in masseter muscle hypertrophy for lower face contouring. Journal of Cosmetic Dermatology, 21 (5), 1849–1856. https://doi.org/10.1111/jocd.14858
- Kroumpouzos, G., Kassir, M., Gupta, M., Patil, A., Goldust, M. (2021). Complications of Botulinum toxin A: An update review. Journal of Cosmetic Dermatology, 20 (6), 1585–1590. https://doi.org/10.1111/jocd.14160
- Park, H. J., Hong, S. O., Kim, H., Oh, W., Kim, H. (2022). Positional deformation of the parotid gland: Application to minimally invasive procedures. Clinical Anatomy, 35 (8), 1147–1151. https://doi.org/10.1002/ca.23941
- Teymoortash, A., Sommer, F., Mandic, R., Schulz, S., Bette, M., Aumüller, G., Werner, J. A. (2007). Intraglandular application of botulinum toxin leads to structural and functional changes in rat acinar cells. British Journal of Pharmacology, 152 (1), 161–167. https://doi.org/10.1038/sj.bjp.0707375
- Ondo, W. G., Hunter, C., Moore, W. (2004). A double-blind placebo-controlled trial of botulinum toxin B for sialorrhea in Parkinson’s disease. Neurology, 62 (1), 37–40. https://doi.org/10.1212/01.wnl.0000101713.81253.4c
- Jackson, C. E., Gronseth, G., Rosenfeld, J., Barohn, R. J., Dubinsky, R., Simpson, C. B. et al. (2009). Randomized double‐blind study of botulinum toxin type B for sialorrhea in als patients. Muscle & Nerve, 39 (2), 137–143. https://doi.org/10.1002/mus.21213
- Greene, P., Fahn, S., Diamond, B. (1994). Development of resistance to botulinum toxin type A in patients with torticollis. Movement Disorders, 9 (2), 213–217. Portico. https://doi.org/10.1002/mds.870090216
- Dressler, D., Tacik, P., Adib Saberi, F. (2013). Botulinum toxin therapy of cervical dystonia: comparing onabotulinumtoxinA (Botox®) and incobotulinumtoxinA (Xeomin®). Journal of Neural Transmission, 121 (1), 29–31. https://doi.org/10.1007/s00702-013-1076-z
- Sethi, K. D., Rodriguez, R., Olayinka, B. (2012). Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. Journal of Medical Economics, 15 (3), 419–423. https://doi.org/10.3111/13696998.2011.653726
- Fernandez, H. H., Evidente, V. G. H., Truong, D., Brodsky, M., Hanschmann, A., Comella, C. L., Jankovic, J. (2013). Long-term treatment of blepharospasm and cervical dystonia: Incobotulinum toxin A is well tolerated when injected at flexible intervals based on patient needs. Journal of the Neurological Sciences, 333, e120. https://doi.org/10.1016/j.jns.2013.07.403
- Botox® (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use (2020). Allergan Inc. Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/103000s5317lbl Last accessed: 30.04.2024
- Dressler, D., Adib Saberi, F. (2005). Botulinum Toxin: Mechanisms of Action. European Neurology, 53 (1), 3–9. https://doi.org/10.1159/000083259
- Dayan, S. H., Maas, C. S. (2007). Botulinum Toxins for Facial Wrinkles: Beyond Glabellar Lines. Facial Plastic Surgery Clinics of North America, 15 (1), 41–49. https://doi.org/10.1016/j.fsc.2006.12.001
- Alam, M., Dover, J. S., Arndt, K. A. (2002). Pain Associated With Injection of Botulinum A Exotoxin Reconstituted Using Isotonic Sodium Chloride With and Without Preservative. Archives of Dermatology, 138 (4), 510. https://doi.org/10.1001/archderm.138.4.510
- Klein, A. W. (2003). Complications, Adverse Reactions, and Insights With the Use of Botulinum Toxin. Dermatologic Surgery, 29 (5), 549–556. https://doi.org/10.1046/j.1524-4725.2003.29129.x
- Thaller, S. R., Kim, S. (1998). The use of botulinum toxin to improve results in aesthetic facial surgery. Aesthetic Plastic Surgery, 22 (2), 141–147.
- Carruthers, J. D., Lowe, N. J., Menter, M. A., Gibson, J., Eadie, N. (2003). Double-Blind, Placebo-Controlled Study of the Safety and Efficacy of Botulinum Toxin Type A for Patients with Glabellar Lines. Plastic and Reconstructive Surgery, 112 (4), 1089–1098. https://doi.org/10.1097/01.prs.0000076504.79727.62
- Dayan, S. H., Maas, C. S. (2007). Botulinum Toxins for Facial Wrinkles: Beyond Glabellar Lines. Facial Plastic Surgery Clinics of North America, 15 (1), 41–49. https://doi.org/10.1016/j.fsc.2006.12.001
- Gart, M. S., Gutowski, K. A. (2016). Overview of Botulinum Toxins for Aesthetic Uses. Clinics in Plastic Surgery, 43 (3), 459–471. https://doi.org/10.1016/j.cps.2016.03.003
- Cula, G. O., Bargo, P. R., Nkengne, A., Kollias, N. (2012). Assessing facial wrinkles: automatic detection and quantification. Skin Research and Technology, 19 (1), 243–251. https://doi.org/10.1111/j.1600-0846.2012.00635.x
- Simon, C. et al. (2010). Botulinum Toxin for Cosmetic. UTMB Health, 122.
- Dayan, S. H. (2013). Complications from Toxins and Fillers in the Dermatology Clinic. Facial Plastic Surgery Clinics of North America, 21 (4), 663–673. https://doi.org/10.1016/j.fsc.2013.07.008
- Alam, M., Tung, R. (2018). Injection technique in neurotoxins and fillers: Indications, products, and outcomes. Journal of the American Academy of Dermatology, 79 (3), 423–435. https://doi.org/10.1016/j.jaad.2018.01.037
- Hassouneh, B., Newman, J. P. (2013). Laser, fillers, and neurotoxins avoiding complication in the cosmetic facial practice. Facial Plastic Surgery Clinics of North America, 21 (4), 585–598. https://doi.org/10.1016/j.fsc.2013.07.002
- Bellomo, R., Harrington, L. K. (2016). Neurotoxins and Dermal Fillers: choosing the right product. Physician Assistant Clinics, 1 (2), 333–345. https://doi.org/10.1016/j.cpha.2015.12.008
- Niamtu, J. (2009). Complications in Fillers and Botox. Oral and Maxillofacial Surgery Clinics of North America, 21 (1), 13–21. https://doi.org/10.1016/j.coms.2008.11.001
- Kim, K., Jeon, S., Kim, J.-K., Hwang, J. S. (2015). Effects of Kyunghee Facial Resistance Program (KFRP) on mechanical and elastic properties of skin. Journal of Dermatological Treatment, 27 (2), 191–196. https://doi.org/10.3109/09546634.2015.1056078
- Ezure, T., Hosoi, J., Amano, S., Tsuchiya, T. (2009). Sagging of the cheek is related to skin elasticity, fat mass and mimetic muscle function. Skin Research and Technology, 15 (3), 299–305. https://doi.org/10.1111/j.1600-0846.2009.00364.x
- Castillo-Garzón, M. J., Ruiz, J. R., Ortega, F. B., Gutiérrez, Á. (2006). Anti-aging therapy through fitness enhancement. Clinical Interventions in Aging, 1 (3), 213–220. https://doi.org/10.2147/ciia.2006.1.3.213
- Clark, H. M., O’Brien, K., Calleja, A., Newcomb Corrie, S. (2009). Effects of Directional Exercise on Lingual Strength. Journal of Speech, Language, and Hearing Research, 52 (4), 1034–1047. https://doi.org/10.1044/1092-4388(2009/08-0062)
- Plastic Surgery Statistics Report (2018). Available at: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf Last accessed: 30.04.2024
- Schlessinger, J., Monheit, G., Kane, M. A. C., Mendelsohn, N. (2011). Time to Onset of Response of AbobotulinumtoxinA in the Treatment of Glabellar Lines: A Subset Analysis of Phase 3 Clinical Trials of a New Botulinum Toxin Type A. Dermatologic Surgery, 37 (10), 1434–1442. https://doi.org/10.1111/j.1524-4725.2011.02075.x
- Rappl, T., Parvizi, Wiedner, May, Kranzelbinder, Friedl, H., Wurzer, P. (2013). Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double-blind study. Clinical, Cosmetic and Investigational Dermatology, 6, 211–219. https://doi.org/10.2147/ccid.s41537
- Flynn, T. C. (2007). Botox in men. Dermatologic Therapy, 20 (6), 407–413. https://doi.org/10.1111/j.1529-8019.2007.00156.x
- Flynn, T. C. (2010). Botulinum toxin: examining duration of effect in facial aesthetic applications. American Journal of Clinical Dermatology, 11 (3), 183–199. https://doi.org/10.2165/11530110-000000000-00000
- Fedok, F. (1996). The Aging Face. Facial Plastic Surgery, 12 (2), 107–115. https://doi.org/10.1055/s-0028-1082402
- Sundaram, H., Liew, S., Signorini, M., Vieira Braz, A., Fagien, S., Swift, A. et al. (2016). Global Aesthetics Consensus: Hyaluronic Acid Fillers and Botulinum Toxin Type A – Recommendations for Combined Treatment and Optimizing Outcomes in Diverse Patient Populations. Plastic & Reconstructive Surgery, 137 (5), 1410–1423. https://doi.org/10.1097/prs.0000000000002119
- Carruthers, J., Burgess, C., Day, D., Fabi, S. G., Goldie, K., Kerscher, M. et al. (2016). Consensus Recommendations for Combined Aesthetic Interventions in the Face Using Botulinum Toxin, Fillers, and Energy-Based Devices. Dermatologic Surgery, 42 (5), 586–597. https://doi.org/10.1097/dss.0000000000000754
- Dover, J. S., Monheit, G., Greener, M., Pickett, A. (2018). Botulinum Toxin in Aesthetic Medicine: Myths and Realities. Dermatologic Surgery, 44 (2), 249–260. https://doi.org/10.1097/dss.0000000000001277
- Rogozhin, A. A., Pang, K. K., Bukharaeva, E., Young, C., Slater, C. R. (2008). Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin A. The Journal of Physiology, 586 (13), 3163–3182. https://doi.org/10.1113/jphysiol.2008.153569
- Courtney, J., Steinbach, J. H. (1981). Age changes in neuromuscular junction morphology and acetylcholine receptor distribution on rat skeletal muscle fibres. The Journal of Physiology, 320 (1), 435–447. https://doi.org/10.1113/jphysiol.1981.sp013960
- Gonzalez-Freire, M., de Cabo, R., Studenski, S. A., Ferrucci, L. (2014). The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Frontiers in Aging Neuroscience, 6. https://doi.org/10.3389/fnagi.2014.00208
- Cheng, C. M. (2007). Cosmetic use of botulinum toxin type A in the elderly. Clinical Interventions in Aging, 2 (1), 81–83. https://doi.org/10.2147/ciia.2007.2.1.81
- Yamauchi, P. (2010). Selection and preference for botulinum toxins in the management of photoaging and facial lines: patient and physician considerations. Patient Preference and Adherence, 4, 345–354. https://doi.org/10.2147/ppa.s6494
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Ольга Володимирівна Філіпцова, Ольга Іванівна Набока, Наталя Вікторівна Хохленкова, Катерина Юріївна Калашнік, Ольга Сергіївна Калюжная, Наталія Власівна Двінських, Аліна Володимирівна Соловйова, Андрій Вікторович Захар’єв, Людмила Станіславівна Петровська

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.







