Effectiveness of a new combined remedy based on a derivative of dicarbonic acid in conditions of drug-induced hepatitis
DOI:
https://doi.org/10.15587/2519-8025.2024.320788Keywords:
liver damage, drug-induced hepatitis, paracetamol, enzyme activity, sialic acids, histological examinationAbstract
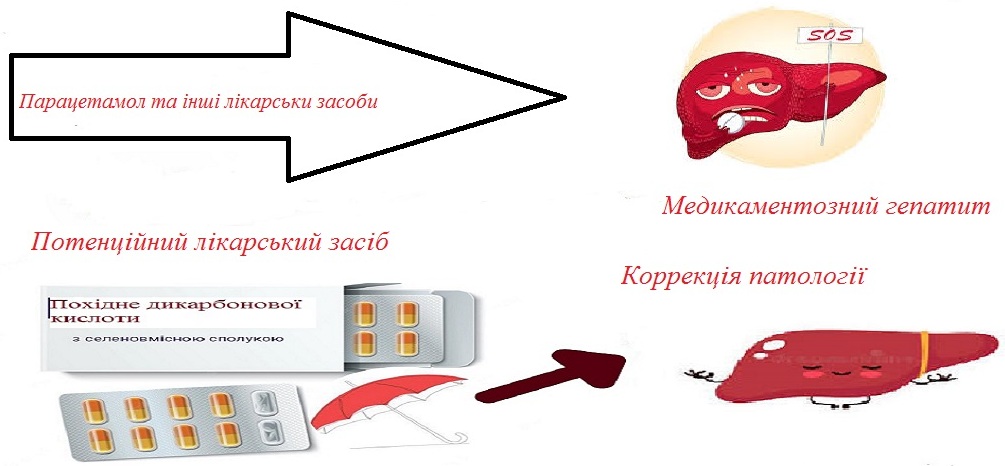
The aim of the study is to evaluate the effect of a new combined remedy based on dicarboxylic acid and a selenium-containing compound on the functional and metabolic state of the liver in conditions of prolonged paracetamol-induced hepatitis.
Materials and methods The study was conducted on outbred white rats weighing 200-250 g in compliance with all principles of ethical treatment of animals. Drug-induced liver injury was modeled by oral administration of paracetamol per os for four weeks at a dose of 600 mg per kg body weight. Animals were divided into groups: I - control, which was injected with a solvent (2 % starch solution); II - rats with paracetamol-induced hepatitis; III - rats with hepatitis, which received a new agent based on a dicarboxylic acid derivative and a selenium-containing compound at a dose of 32 mg/kg b.w. Verification of functional and metabolic disorders of the liver was carried out using biochemical methods and histological examination of the liver tissue.
Results Significant changes in the activity of hepatic enzymes in animals from the induced hepatitis group compared to intact control animals, increased levels of sialic acids in the blood serum and liver, etc. were recorded. Structural changes in the liver tissue of animals with induced hepatitis were also observed compared to intact control animals. The use of the new remedy led to the normalization of the indicators.
Conclusions. In the conditions of paracetamol-induced hepatitis, changes in biochemical parameters in the blood serum and liver homogenate were observed, indicating the development of autoimmune processes in the body. Correction with the new remedy led to the restoration of liver enzyme activity and a decrease in the content of sialic acids in the blood serum and liver homogenate of experimental animals. The new combined remedy based on a dicarboxylic acid derivative and a selenium-containing compound is effective and promising as a potential drug for the correction of liver disorders in the setting of drug-induced hepatitis
References
- Rotundo, L., Pyrsopoulos, N. (2020). Liver injury induced by paracetamol and challenges associated with intentional and unintentional use. World Journal of Hepatology, 12 (4), 125–136. https://doi.org/10.4254/wjh.v12.i4.125
- Reuben, A., Koch, D. G., Lee, W. M. (2010). Drug-induced acute liver failure: Results of a U.S. multicenter, prospective study. Hepatology, 52 (6), 2065–2076. https://doi.org/10.1002/hep.23937
- Watkins, P. B., Kaplowitz, N., Slattery, J. T., Colonese, C. R., Colucci, S. V., Stewart, P. W., Harris, S. C. (2006). Aminotransferase Elevations in Healthy Adults Receiving 4 Grams of Acetaminophen Daily: a randomized controlled trial. JAMA, 296 (1), 87–93. https://doi.org/10.1001/jama.296.1.87
- Mooli, R. G. R., Mukhi, D., Ramakrishnan, S. K. (2022). Oxidative Stress and Redox Signaling in the Pathophysiology of Liver Diseases. Comprehensive Physiology, 12 (2), 3167–3192. https://doi.org/10.1002/cphy.c200021
- Allaire, M., Rautou, P.-E., Codogno, P., Lotersztajn, S. (2019). Autophagy in liver diseases: Time for translation? Journal of Hepatology, 70 (5), 985–998. https://doi.org/10.1016/j.jhep.2019.01.026
- Kudria, M. Ya., Melnykivska, N. V., Ustenko, N. V. et al. (2015). Hepatoprotektorna aktyvnist stymuliatora spermatoheneza – katiazynu za umov eksperymentalnoi paratsetamol-indukovanoi patolohii pechinky. Problemy endokrynnoi patolohii, 4, 53–60.
- Stefanov, A. V. (Ed.) (2002). Doklinicheskie issledovaniia lekarstvennykh sredstv. Kiiv: Avitcenna, 568.
- Nair, A., Jacob, S. (2016). A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy, 7 (2), 27–31. https://doi.org/10.4103/0976-0105.177703
- Yaremenko, F. H., Svydlo, I. M., Vakula, V. M. et al. (2001). Pat. No. 38/30 A UA. 3 (4,5-dyhidrotiazol-2-il) amid tsys-1,2,2-trymetyltsyklopentan-1,3-dykarbonovoi kysloty, shcho stymuliuie spermatohenez. MPK 7 A 61 K 31/16; SO7S 61/06; SO7D 277/00. No. 2000083139; declareted: 01.06.00; published: 13.05.01, Bul. No. No. 4, 159.
- Instruktsiia do naboru reaktyviv dlia vyznachennia aktyvnosti aspartat-alaninamynotransferaz (AsT – AlT 120) v syrovattsi krovi (metod Raitmana-Frenkelia) (2021). Filisit-Diahnostyka, 4.
- Instruktsiia do naboru reaktyviv dlia vyznachennia aktyvnosti hamahlutamil-transpeptydazy (γ-HHT) u syrovattsi krovi kinetychnym metodom (2021). SpainLab, 4.
- Instruktsiia do naboru reaktyviv dlia vyznachennia luzhnoi fosfatazy u syrovattsi krovi kinetychnym metodom (2021). SpainLab, 4.
- Instruktsiia do naboru reaktyviv dlia vyznachennia sialovykh kyslot u biolohichnykh ridynakh kolorymetrychnym metodom (2021). Filisit-Diahnostyka, 4.
- Kiernan, J. A. (2015). Histological and histochemical methods: theory and practice. Banbury: Scion Publishing, 571.
- Atramentova, L. A., Utevskaia, O. M. (2008). Statisticheskie metody v biologii. Gorlovka: Vid-vo Lіkhtar, 248.
- Herndon, C. M., Dankenbring, D. M. (2014). Patient Perception and Knowledge of Acetaminophen in a Large Family Medicine Service. Journal of Pain & Palliative Care Pharmacotherapy, 28 (2), 109–116. https://doi.org/10.3109/15360288.2014.908993
- Moroziuk, A. Yu., Melnykivska, N. V., Kudria, M. Ya., Ustenko, N. V., Kustova, S. P., Boiko, M. O. et al. (2023). Comorbid endocrine pathology as a result of functional and metabolic disorders of the liver and its correction with a new drug. Pharmacology and Drug Toxicology, 17 (5), 318–327. https://doi.org/10.33250/17.04.318
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Анастасія Юріївна Морозюк, Марія Яківна Кудря, Наталя Викторівна Мельниківська, Нонна Василівна Устенко, Юлія Борисівна Лар’яновська

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.








