Assessment of the informativeness of serological methods for the diagnosis of infectious mononucleosis and seroepidemiological data on its prevalence
DOI:
https://doi.org/10.15587/2519-4798.2025.348424Keywords:
infectious mononucleosis, Epstein-Barr virus, serological diagnostics, VCA IgM, VCA IgG, EBNA IgG, monospot test, EBV PCRAbstract
The aim. To assess the prevalence of markers of the acute period of infectious mononucleosis (VCA IgM, VCA IgG, EBNA IgG, heterophilic antibodies in the monospot test and EBV DNA by PCR) based on the analysis of the Dila laboratory database in Kyiv during the three-year observation period (2022-2024), to investigate changes in diagnostic approaches in clinical practice, to determine the effectiveness of various laboratory methods in the context of differential diagnosis with SARS-like diseases and to develop optimized recommendations for the rational use of diagnostic resources.
Materials and methods. A retrospective analysis of 64,812 laboratory tests performed during 2022-2024 in a single network of Kyiv laboratories was conducted. The analysis included serological tests for IgM antibodies to EBV capsid antigen, IgG to capsid antigen (VCA), IgG to nuclear antigen (EBNA), monospot test and EBV PCR. Statistical research methods.
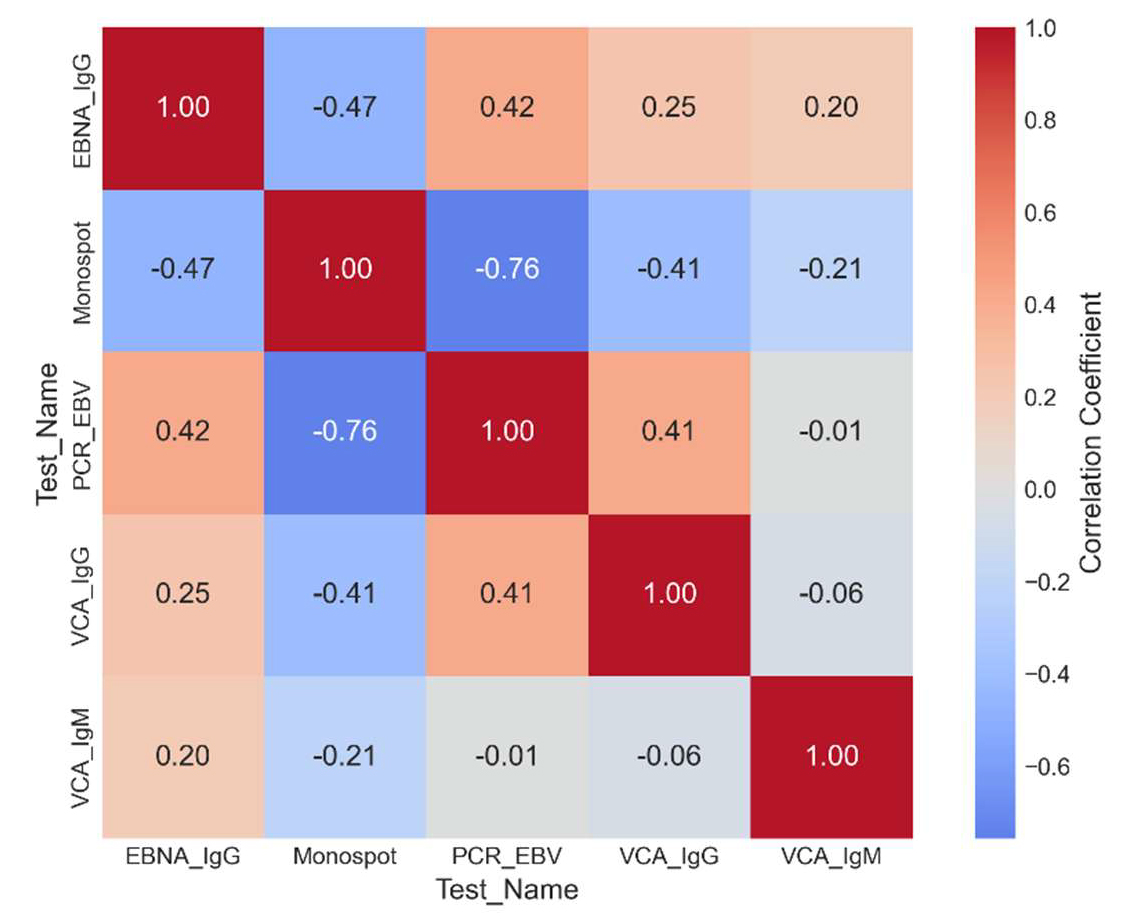
Results. The proportion of positive VCA IgM results remained stable throughout the three years of the study, fluctuating within 15.2-15.8%, which confirms the reliability of this marker of infectious mononucleosis. Serological indicators demonstrated high stability with low coefficients of variation - 4.3% for VCA IgM, 5.2% for VCA IgG and 6.5% for EBNA IgG. In contrast, EBV PCR revealed significant variability (coefficient of variation 118%) with a sharp decrease in positive results from 5.1% to 0.2%. A reorientation of the diagnostic practice of doctors from molecular to serological methods was also revealed - the proportion of serological tests increased from 65.5% to 71.4%, while molecular tests decreased from 34.5% to 28.6%. No seasonal fluctuations in the frequency of acute MI were detected (p=0.153), i.e. infectious mononucleosis was diagnosed equally throughout the year.
Conclusions. VCA IgM should be used as the main marker for the diagnosis of the acute phase of MI. VCA IgG and EBNA IgG are important diagnostic elements for determining the stage of the infectious process. The use of PCR should be limited to cases with an atypical course of the disease or in clinically ambiguous situations
References
- St. Sauver, J. L., Jacobson, R. M., Weston, S. A., Fan, C., Buck, P. O., Hall, S. A. (2025). Population-Based Incidence of Infectious Mononucleosis and Related Hospitalizations: 2010 Through 2021. Mayo Clinic Proceedings, 100 (6), 982–992. https://doi.org/10.1016/j.mayocp.2024.09.017
- Balfour, H. H., Sifakis, F., Sliman, J. A., Knight, J. A., Schmeling, D. O., Thomas, W. (2013). Age-Specific Prevalence of Epstein–Barr Virus Infection Among Individuals Aged 6-19 Years in the United States and Factors Affecting Its Acquisition. The Journal of Infectious Diseases, 208 (8), 1286–1293. https://doi.org/10.1093/infdis/jit321
- Rostgaard, K., Balfour, H. H., Jarrett, R., Erikstrup, C., Pedersen, O., Ullum, H. et al. (2019). Primary Epstein-Barr virus infection with and without infectious mononucleosis. PLOS ONE, 14 (12), e0226436. https://doi.org/10.1371/journal.pone.0226436
- Womack, J., Jimenez, M. (2015). Common questions about infectious mononucleosis. American Family Physician, 91 (6), 372–376.
- Ebell, M. H., Call, M., Shinholser, J., Gardner, J. (2016). Does This Patient Have Infectious Mononucleosis? The rational clinical examination systematic review. JAMA, 315 (14), 1502–1509. https://doi.org/10.1001/jama.2016.2111
- De Paschale, M., Clerici, P. (2012). Serological diagnosis of Epstein-Barr virus infection: Problems and solutions. World Journal of Virology, 1 (1), 31–43. https://doi.org/10.5501/wjv.v1.i1.31
- Laboratory testing for Epstein-Barr virus (EBV) (2024). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/epstein-barr/php/laboratories/index.html
- Robertson, P., Beynon, S., Whybin, R., Brennan, C., Vollmer‐Conna, U., Hickie, I., Lloyd, A. (2003). Measurement of EBV‐IgG anti‐VCA avidity aids the early and reliable diagnosis of primary EBV infection. Journal of Medical Virology, 70 (4), 617–623. Portico. https://doi.org/10.1002/jmv.10439
- Portet Sulla, V., Kadi, A., Mouna, L., Fenaux, H., Cechura, H., Rafek, R. et al. (2024). Investigation of atypical serological profiles for Epstein-Barr virus (EBV). Journal of Virological Methods, 329, 115002. https://doi.org/10.1016/j.jviromet.2024.115002
- Jhaveri, T. A., Harris, C., Sax, P. E. (2022). IgM Positivity for Both EBV and CMV: A Clinical Conundrum. Open Forum Infectious Diseases, 9 (7). https://doi.org/10.1093/ofid/ofac316
- Marshall-Andon, T., Heinz, P. (2017). How to use … the Monospot and other heterophile antibody tests. Archives of Disease in Childhood – Education & Practice Edition, 102 (4), 188–193. https://doi.org/10.1136/archdischild-2016-311526
- Stuempfig, N., Seroy, J. (2023). Monospot test. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK539739/
- Guerrero-Ramos, A., Patel, M., Kadakia, K., Haque, T. (2014). Performance of the Architect EBV Antibody Panel for Determination of Epstein-Barr Virus Infection Stage in Immunocompetent Adolescents and Young Adults with Clinical Suspicion of Infectious Mononucleosis. Clinical and Vaccine Immunology, 21 (6), 817–823. https://doi.org/10.1128/cvi.00754-13
- Gulley, M. L., Tang, W. (2008). Laboratory Assays for Epstein-Barr Virus-Related Disease. The Journal of Molecular Diagnostics, 10 (4), 279–292. https://doi.org/10.2353/jmoldx.2008.080023
- Rowe, D. T., Qu, L., Reyes, J., Jabbour, N., Yunis, E., Putnam, P., Todo, S., Green, M. (1997). Use of quantitative competitive PCR to measure Epstein-Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. Journal of Clinical Microbiology, 35 (6), 1612–1615. https://doi.org/10.1128/jcm.35.6.1612-1615.1997
- Green, M., Michaels, M. G., Katz, B. Z., Burroughs, M., Gerber, D., Shneider, B. L. et al. (2006). CMV-IVIG for Prevention of Epstein Barr Virus Disease and Posttransplant Lymphoproliferative Disease in Pediatric Liver Transplant Recipients. American Journal of Transplantation, 6 (8), 1906–1912. https://doi.org/10.1111/j.1600-6143.2006.01394.x
- Heilman, J., Thurley, N. (2021). Infectious mononucleosis: Diagnosis and treatment. Emergency Care BC. Available at: https://emergencycarebc.ca/clinical_resource/clinical-summary/infectious-mononucleosis-diagnosis-and-treatment/
- Leung, A. K. C., Lam, J. M., Barankin, B. (2024). Infectious Mononucleosis: An Updated Review. Current Pediatric Reviews, 20 (3), 305–322. https://doi.org/10.2174/1573396320666230801091558
- Jain, P., Basnet, S., Syed, S., Arakaki, B., Mues, K. E., Marcum, Z. A., Diaz-Decaro, J. (2023). Testing for Cytomegalovirus Among Individuals Who Were Immunocompromised, 2018-2022. JAMA Network Open, 6 (11), e2345126. https://doi.org/10.1001/jamanetworkopen.2023.45126
- Sylvester, J. E., Buchanan, B. K., Silva, T. W. (2023). Infectious Mononucleosis: Rapid Evidence Review. American Family Physician, 107 (1), 71–78.
- Naughton, P., Healy, M., Enright, F., Lucey, B. (2021). Infectious Mononucleosis: diagnosis and clinical interpretation. British Journal of Biomedical Science, 78 (3), 107–116. https://doi.org/10.1080/09674845.2021.1903683
- Shi, T., Huang, L., Luo, L., Yu, Q., Tian, J. (2020). Diagnostic value of serological and molecular biological tests for infectious mononucleosis by EBV in different age stages and course of the disease. Journal of Medical Virology, 93 (6), 3824–3834. https://doi.org/10.1002/jmv.26558
- Wang, E. X., Kussman, A., Hwang, C. E. (2021). Use of Monospot Testing in the Diagnosis of Infectious Mononucleosis in the Collegiate Student–Athlete Population. Clinical Journal of Sport Medicine, 32 (5), 467–470. https://doi.org/10.1097/jsm.0000000000000996
- Crawford, D. H., Ando, I. (2015). The epidemiology of EBV and its association with malignant disease. Human Herpesviruses. National Center for Biotechnology Information. Available at: https://www.ncbi.nlm.nih.gov/books/NBK47424/
- Lee, A. W. M., Lee, V. H. F., Ng, W.-T., Strojan, P., Saba, N. F. et al. (2021). A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. European Journal of Cancer, 153, 109–122. https://doi.org/10.1016/j.ejca.2021.05.022
- Liu, W., Chen, G., Gong, X., Wang, Y., Zheng, Y., Liao, X. et al. (2021). The diagnostic value of EBV-DNA and EBV-related antibodies detection for nasopharyngeal carcinoma: a meta-analysis. Cancer Cell International, 21 (1). https://doi.org/10.1186/s12935-021-01862-7
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nataliia Dziubenko, Olga Golubovska

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.
Authors, who are published in this journal, agree to the following conditions:
1. The authors reserve the right to authorship of the work and pass the first publication right of this work to the journal under the terms of a Creative Commons CC BY, which allows others to freely distribute the published research with the obligatory reference to the authors of the original work and the first publication of the work in this journal.
2. The authors have the right to conclude separate supplement agreements that relate to non-exclusive work distribution in the form in which it has been published by the journal (for example, to upload the work to the online storage of the journal or publish it as part of a monograph), provided that the reference to the first publication of the work in this journal is included.








