Development of a new solution for determining the solubility limit of quercetin and other poorly soluble substances in aqueous solutions using the method for determining total organic carbon
DOI:
https://doi.org/10.15587/2519-4852.2023.286639Keywords:
quercetin, identification, quantitation, total organic carbon, method development, bioequivalence, biowaiver, solubility, dissolution testAbstract
Aim. Given the incompleteness of literature data on the solubility of quercetin and the importance of this physicochemical characteristic in the study of its bioavailability, there is a need to develop an alternative method for accurately quantifying the solubility limit of quercetin.
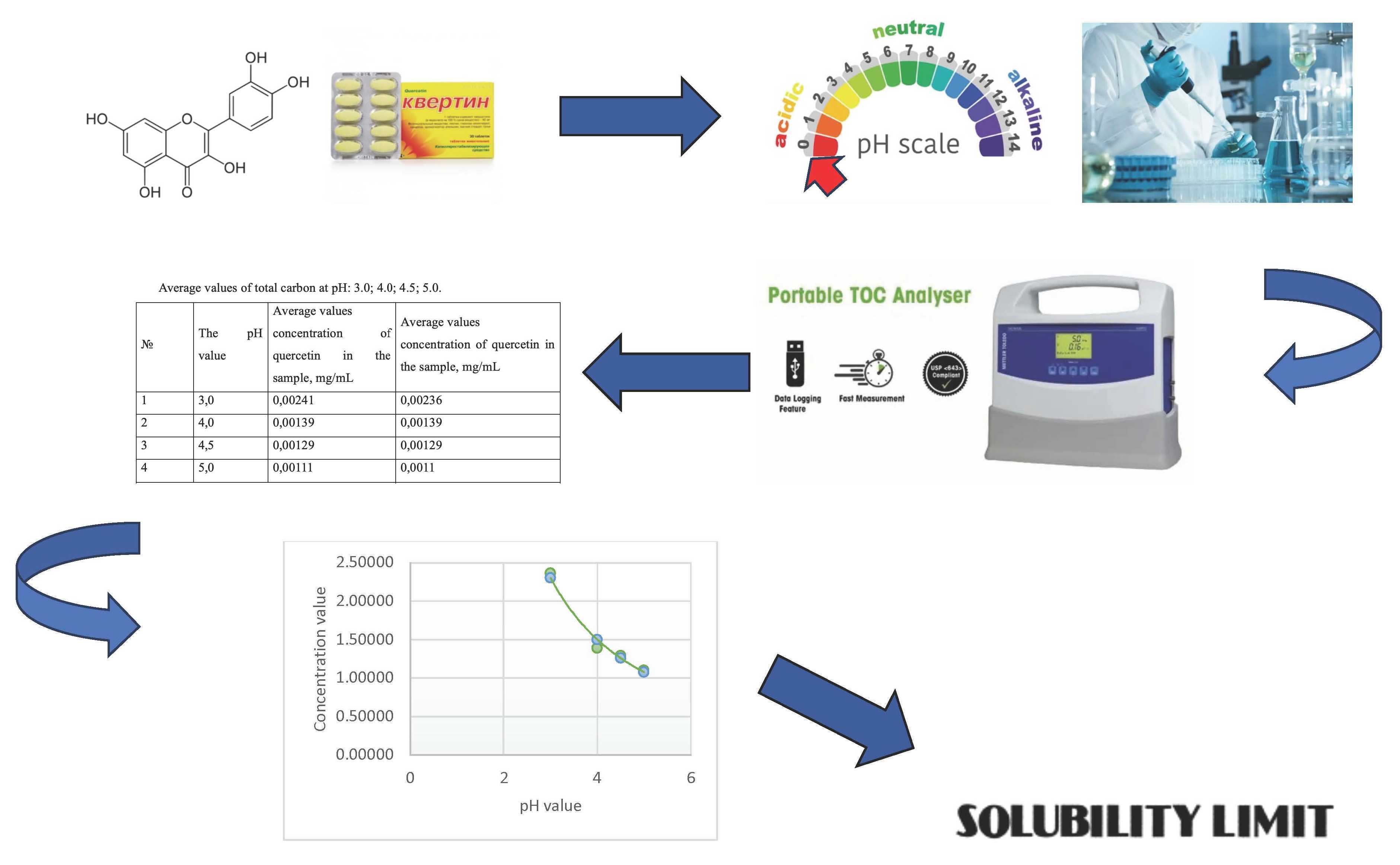
Materials and methods. The concentration of quercetin in the samples was determined by directly determining the total organic carbon. For measurements, a total organic carbon analyzer 450 TOC (METTLER TOLEDO) was used with a range of measured values of 0.05–1000 ppbC µgC/l.
Results. The exact limit of the solubility of quercetin, as a poorly soluble substance, has been established. Having previously measured the value of total organic carbon in the prepared solutions, we obtained data on the concentration of quercetin in solutions depending on the pH of the solution.
Having built a graphical dependence of the measured values of the concentration of a substance on the pH values of the studied solutions, we obtain a mathematical equation of the reliance. Using the resulting function equation, one can approximate the concentration value of a substance with a pH value of 7.0. This value will be the solubility limit of the test substance for neutral media.
Conclusions. As a result of the research, a new method was proposed for the quantitative determination of the solubility limit of a substance, with an accuracy not exceeding 5.0 %. The method is based on measuring the concentration of total carbon in acidic solutions with different pH values and subsequent approximation of the obtained dependence of the pH value equal to 7.0
References
- Pal, D. K., Verma, P. (2013). Flavonoids: A powerful and abundant source of antioxidants. International Journal of Pharmaceutical Sciences and Research, 5, 95–98.
- Tutelian, V. A., Lashneva, N. V. (2013). Biologically active substances of plant origin. Flavonols and flavones: Prevalence, dietary sources, and consumption. Voprosy Pitaniia, 82, 4–22.
- Lakhanpal, P., Rai, D. K. (2007). Quercetin: A Versatile Flavonoid. Internet Journal of Medical Update, 2 (2), 22–37. doi: https://doi.org/10.4314/ijmu.v2i2.39851
- Jung, J.-H., Kang, J.-I., Kim, H.-S. (2012). Effect of quercetin on impaired immune function in mice exposed to irradiation. Nutrition Research and Practice, 6 (4), 301–307. doi: https://doi.org/10.4162/nrp.2012.6.4.301
- Xiao, X., Shi, D., Liu, L., Wang, J., Xie, X., Kang, T., Deng, W. (2011). Quercetin Suppresses Cyclooxygenase-2 Expression and Angiogenesis through Inactivation of P300 Signaling. PLoS ONE, 6 (8), e22934. doi: https://doi.org/10.1371/journal.pone.0022934
- Liu, H., Zhang, L., Lu, S. (2012). Evaluation of Antioxidant and Immunity Activities of Quercetin in Isoproterenol-Treated Rats. Molecules, 17 (4), 4281–4291. doi: https://doi.org/10.3390/molecules17044281
- Lee, K. M., Hwang, M. K., Lee, D. E., Lee, K. W., Lee, H. J. (2010). Protective Effect of Quercetin against Arsenite-Induced COX-2 Expression by Targeting PI3K in Rat Liver Epithelial Cells. Journal of Agricultural and Food Chemistry, 58 (9), 5815–5820. doi: https://doi.org/10.1021/jf903698s
- Dong, Y., Wang, J., Feng, D., Qin, H., Wen, H., Yin, Z. et al. (2014). Protective Effect of Quercetin against Oxidative Stress and Brain Edema in an Experimental Rat Model of Subarachnoid Hemorrhage. International Journal of Medical Sciences, 11 (3), 282–290. doi: https://doi.org/10.7150/ijms.7634
- Agrawal, A. D. (2011). Pharmacological Activities of Flavonoids: A Review. International Journal of Pharmaceutical Sciences and Nanotechnology, 4 (2), 1394–1398. doi: https://doi.org/10.37285/ijpsn.2011.4.2.3
- Vauzour, D., Vafeiadou, K., Rodriguez-Mateos, A., Rendeiro, C., Spencer, J. P. E. (2008). The neuroprotective potential of flavonoids: a multiplicity of effects. Genes & Nutrition, 3 (3-4), 115–126. doi: https://doi.org/10.1007/s12263-008-0091-4
- Salvamani, S., Gunasekaran, B., Shaharuddin, N. A., Ahmad, S. A., Shukor, M. Y. (2014). Antiartherosclerotic Effects of Plant Flavonoids. BioMed Research International, 2014, 1–11. doi: https://doi.org/10.1155/2014/480258
- Denny Joseph, K. M., Muralidhara. (2013). Enhanced neuroprotective effect of fish oil in combination with quercetin against 3‐nitropropionic acid induced oxidative stress in rat brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 40, 83–92. doi: https://doi.org/10.1016/j.pnpbp.2012.08.018
- Parasuraman, S., Maithili, K. S. (2014). Antioxidant and drug metabolism. Free Radicals and Antioxidants, 4 (1), 1–2. doi: https://doi.org/10.5530/fra.2014.1.1
- Procházková, D., Boušová, I., Wilhelmová, N. (2011). Antioxidant and prooxidant properties of flavonoids. Fitoterapia, 82 (4), 513–523. doi: https://doi.org/10.1016/j.fitote.2011.01.018
- Li, Y., Yao, J., Han, C., Yang, J., Chaudhry, M., Wang, S. et al. (2016). Quercetin, Inflammation and Immunity. Nutrients, 8 (3), 167. doi: https://doi.org/10.3390/nu8030167
- Salehi, B., Machin, L., Monzote, L., Sharifi-Rad, J., Ezzat, S. M., Salem, M. A. et al. (2020). Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega, 5 (20), 11849–11872. doi: https://doi.org/10.1021/acsomega.0c01818
- Nastanova z klinichnykh doslidzhen «Likarski zasoby. Doslidzhennia biodostupnosti ta bioekvivalentnosti» (Nastanova 42–7.1:2005) (2005). Kyiv: Ministerstvo okhorony zdorov’ia Ukrainy.
- Khanina, N., Georgiyants, V., Khanin, V. (2023). Development of a method for the quantitative determination of the solubility limits of poorly soluble in water substances on the example of quercetin. ScienceRise: Pharmaceutical Science, 3 (43), 58–66. doi: https://doi.org/10.15587/2519-4852.2023.283293
- PubChem. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin
- Derzhavna Farmakopeia Ukrainy. Vol. 1. Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1, 1128.
- Epshtein, N. A. (2019). Validation of Analytical Procedures: Graphic and Calculated Criteria for Assessment of Methods Linearity in Practice. Drug Development & Registration, 8 (2), 122–130. doi: https://doi.org/10.33380/2305-2066-2019-8-2-122-130
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Nataliia Khanina, Victoriya Georgiyants, Vadim Khanin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






