Study of chondroprotective properties of interleukin-1 receptor antagonist.
DOI:
https://doi.org/10.15587/2519-4852.2024.298740Keywords:
interleukin-1 receptor antagonist, raleukin, osteoarthritis, chondroprotective effect, anti-inflammatory effectAbstract
Osteoarthritis is one of the most widespread diseases, represents a medical and socio-economic problem and is one of the first places among the causes of long-term disability of the population in the world. Cytokine mechanisms of osteoarthritis development are attracting more and more attention.
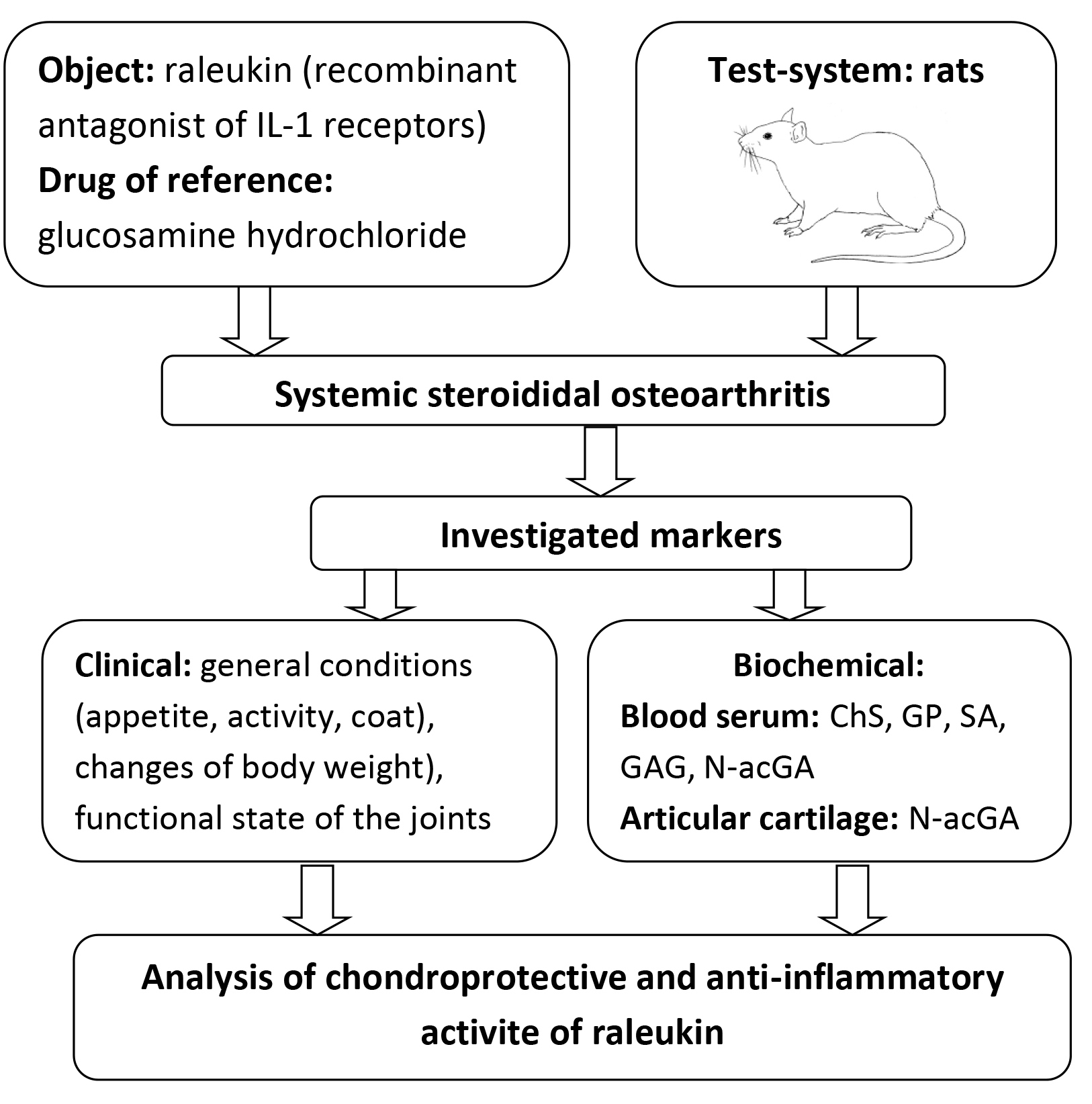
The aim of the study was to determine the chondroprotective and anti-inflammatory properties of the original recombinant interleukin-1 (IL-1) receptor antagonist (ARIL-1) raleukin on the model of systemic steroid osteoarthritis (SSO) in rats.
Materials and methods. The SSO model was reproduced in a modified form by intramuscular three-time administration of dexamethasone at a dose of 7 mg/kg with an interval of one week. Raleukin was injected subcutaneously in a conditionally effective dose of 3 mg/kg for anti-inflammatory activity, and glucosamine (GA) orally in a dose of 50 mg/kg (ED50 for anti-inflammatory activity). Starting from the 28th day of the study and for 4 weeks, the study objects were introduced by the appropriate route once a day.
Result. The results of the experiment show that clinical signs of damage to the locomotor system appeared in all animals after three administrations of dexamethasone. Later and before the end of the experiment, a typical clinical picture of the development of SSO was observed, which was confirmed by the results of the study of biochemical markers (mainly in blood serum) of the state of the connective tissue of the experimental animals.
Significant changes in the functional status of the animals were noted in rats with SSO who received raleukin starting from the second week of administration. In rats, motor activity increased, tolerance to physical exertion increased, joint condition visually normalised, and appetite increased. When the reference drug GA was administered, the functional state of the animals differed from the control pathology group to a somewhat lesser extent. Besides, raleukin did not reliably differ from GA in its effect on biochemical parameters characterising the state of connective tissue and the content of its main metabolites in the blood serum of rats with steroid osteoarthritis.
Conclusions. In the model of systemic steroid osteoarthritis, raleukin contributed to the improvement of functional indicators of the condition of animals and the normalisation of their body weight; namely, it moderately reduced the content of all markers of connective tissue metabolism in the blood serum of animals, especially chondroitin sulfates and sialic acids, which can be explained by the systemic nature of its effect. In terms of its effect on the level of the main metabolites of connective tissue in the blood serum of rats, raleukin prevailed over glucosamine hydrochloride. Thus, the analysis of biochemical data against the background of experimental osteoarthritis allows us to draw a conclusion about the high chondroprotective and anti-inflammatory potential of the recombinant IL-1 receptor antagonist
References
- Yin, F., Yang, Q., He, Y., Peng, L., Zhao, Z., He, C., Chen, J. (2021). Top 100 cited articles on osteoarthritis from 1990 to 2020. Rheumatology and Immunology Research, 2 (4), 241–248. doi: https://doi.org/10.2478/rir-2021-0033
- Cui, A., Li, H., Wang, D., Zhong, J., Chen, Y., Lu, H. (2020). Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine, 29–30, 100587. doi: https://doi.org/10.1016/j.eclinm.2020.100587
- Rodriguez-Veiga, D., González-Martín, C., Pertega-Díaz, S., Seoane-Pillado, T., Barreiro-Quintás, M., Balboa-Barreiro, V. (2023). Prevalence of osteoarthritis of the knee in a random population sample of people aged 40 and older. Gaceta Médica de México, 155 (1), 39–45. doi: https://doi.org/10.24875/gmm.m19000231
- Chen, D., Shen, J., Zhao, W., Wang, T., Han, L., Hamilton, J. L., Im, H.-J. (2017). Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Research, 5 (1). doi: https://doi.org/10.1038/boneres.2016.44
- Kapoor, M., Martel-Pelletier, J., Lajeunesse, D., Pelletier, J.-P., Fahmi, H. (2010). Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Reviews Rheumatology, 7 (1), 33–42. doi: https://doi.org/10.1038/nrrheum.2010.196
- Bedaiwi, M. K., Almaghlouth, I., Omair, M. A. (2021). Effectiveness and adverse effects of anakinra in treatment of rheumatoid arthritis: a systematic review. European Review for Medical and Pharmacological Sciences, 25, 7833–7839. doi: https://doi.org/10.26355/eurrev_202112_27630
- Molnar, V., Matišić, V., Kodvanj, I., Bjelica, R., Jeleč, Ž., Hudetz, D. et al. (2021). Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. International Journal of Molecular Sciences, 22 (17), 9208. doi: https://doi.org/10.3390/ijms22179208
- Scanzello, C. R. (2017). Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. Journal of Orthopaedic Research, 35 (4), 735–739. doi: https://doi.org/10.1002/jor.23471
- Chow, Y. Y., Chin, K.-Y. (2020). The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediators of Inflammation, 2020, 1–19. doi: https://doi.org/10.1155/2020/8293921
- Boraschi, D., Italiani, P., Weil, S., Martin, M. U. (2017). The family of the interleukin‐1 receptors. Immunological Reviews, 281 (1), 197–232. doi: https://doi.org/10.1111/imr.12606
- Jenei-Lanzl, Z., Meurer, A., Zaucke, F. (2019). Interleukin-1β signaling in osteoarthritis – chondrocytes in focus. Cellular Signalling, 53, 212–223. doi: https://doi.org/10.1016/j.cellsig.2018.10.005
- Nikfar, S., Saiyarsarai, P., Tigabu, B. M., Abdollahi, M. (2018). Efficacy and safety of interleukin-1 antagonists in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology International, 38 (8), 1363–1383. doi: https://doi.org/10.1007/s00296-018-4041-1
- Hommel, U., Hurth, K., Rondeau, J.-M., Vulpetti, A., Ostermeier, D., Boettcher, A. et al. (2023). Discovery of a selective and biologically active low-molecular weight antagonist of human interleukin-1β. Nature Communications, 14 (1). doi: https://doi.org/10.1038/s41467-023-41190-0
- Reginster, J. Y., Deroisy, R., Rovati, L. C., Lee, R. L., Lejeune, E., Bruyere, O. et al. (2001). Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. The Lancet, 357 (9252), 251–256. doi: https://doi.org/10.1016/s0140-6736(00)03610-2
- Zupanets, K. O., Shebeko, S. K., Otrishko, I. A. (2010). Doslidzhennia vplyvu kompozytsii na osnovi kvertsetynu ta pokhidnykh hliukozaminu na protsesy apoptozu khondrotsytiv v umovakh rozvytku eksperymentalnoho osteoartrytu. Liky Ukrainy plius, 3 (12), 47–50.
- Zupanets, Y. A., Korzh, N. A., Dedukh, N. V. et al. (1999). Metodycheskye rekomendatsyy po eksperymentalnomu yssledovanyiu y klynycheskomu yzuchenyiu protyvoartroznikh (khondromodulyruiushchykh) lekarstvennikh sredstv. Kyiv, 56.
- Levchenko, V. I., Novozhytskaia, Yu. M., Sakhniuk, V. V. ta in. (2004). Biokhimichni metody doslidzhennia krovi khvorykh: Metodychni rekomendatsii dlia likariv khimiko-toksykolohichnykh viddiliv derzhavnykh laboratorii veterynarnoi medytsyny Ukrainy. Kyiv, 104.
- Shtern, M. R., Tymoshenko, O. P., Leonteva, F. S., Kliueva, H. F. (1982). A. S. No. 960626 SSSR. MPK G0923/28. Sposob opredelenyia hlykozamynohlykansulfatov v sivorotke krovy. published: 23.09.82, Bul. No. 35, 6.
- Leontieva, F. S., Filipenko, V. A., Tymoshenko, O. P., Kartashov, M. I., Kibkalo, D. V., Tuliakov, V. O., Riabkova, L. P. (2008). Pat. No. 29198 UA. MPK G01N33/48. Sposib vyznachennia fraktsii sulfatovanykh heksozaminohlikaniv. No. u 200708505; declareted: 24.07.2007; published: 10.01.2008, Bul. No. 1.
- Zupanets, I. A., Shebeko, S. K. (2005). Unifikatsiia metodiv kilkisnoho vyznachennia endohennoho hliukozaminu u biolohichnomu materiali. Farmakom, 4, 56–61.
- Xie, R., Yao, H., Mao, A. S., Zhu, Y., Qi, D., Jia, Y. et al. (2021). Biomimetic cartilage-lubricating polymers regenerate cartilage in rats with early osteoarthritis. Nature Biomedical Engineering, 5 (10), 1189–1201. doi: https://doi.org/10.1038/s41551-021-00785-y
- Kourí, J. B., Rojas, L., Pérez, E., Abbud-Lozoya, K. A. (2002). Modifications of Golgi Complex in Chondrocytes from Osteoarthrotic (OA) Rat Cartilage. Journal of Histochemistry & Cytochemistry, 50 (10), 1333–1339. doi: https://doi.org/10.1177/002215540205001006
- Korzh, N. A., Dedukh, N. V., Zupantc, I. A. (Eds.) (2007). Osteoartroz: konservativnaia terapiia. Kharkiv: Zolotye stranitcy, 424.
- Palmer, G., Guerne, P.-A., Mezin, F., Maret, M., Guicheux, J., Goldring, M. B., Gabay, C. (2002). Production of interleukin-1 receptor antagonist by human articular chondrocytes. Arthritis Research & Therapy, 4 (3), 226–231. doi: https://doi.org/10.1186/ar411
- Kim, J. E., Song, D., Kim, S. H., Jung, Y., Kim, S. J. (2018). Development and characterization of various osteoarthritis models for tissue engineering. PLOS ONE, 13 (3), e0194288. doi: https://doi.org/10.1371/journal.pone.0194288
- Li, L., Li, Z., Li, Y., Hu, X., Zhang, Y., Fan, P. (2020). Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskeletal Disorders, 21 (1). doi: https://doi.org/10.1186/s12891-020-3120-0
- Dinarello, C. A. (2017). Overview of the IL‐1 family in innate inflammation and acquired immunity. Immunological Reviews, 28 1(1), 8–27. doi: https://doi.org/10.1111/imr.12621
- Ruscitti, P., Masedu, F., Alvaro, S., Airò, P., Battafarano, N., Cantarini, L. et al. (2019). Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLOS Medicine, 16 (9), e1002901. doi: https://doi.org/10.1371/journal.pmed.1002901
- Kovalenko, Ye. M. (2009). Farmakolohichne vyvchennia protyzapalnoi aktyvnosti antahonista retseptoriv interleikina-1 (ARIL-1). Kharkiv, 19.
- Cadet, C., Maheu, E. (2021). Non-steroidal anti-inflammatory drugs in the pharmacological management of osteoarthritis in the very old: prescribe or proscribe? Therapeutic Advances in Musculoskeletal Disease, 13. doi: https://doi.org/10.1177/1759720x211022149
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Kateryna Shchokina, Sergiy Shtrygol’, Sergii Shebeko, Halyna Bielik, Tetiana Kutsenko, Andrii Taran

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






