Semi-solid extrusion 3D printing of functionalized polyethylene oxide gels loaded with 1,2,3-triazolo-1,4-benzodiazepine nanofibers and valine-modified motherwort (Leonurus cardiaca L.) dry extract
DOI:
https://doi.org/10.15587/2519-4852.2024.299205Keywords:
benzodiazepine derivative, motherwort extract, nanofibers, oleogel, polyethylene oxide, 3D printingAbstract
Anxiety disorders are the most prevalent psychiatric disorders and are associated with a high burden of illness. Combining synthetic and native-origin compounds in treating such disorders could provide true benefits in terms of therapeutic efficacy. In the present study, we combined triazolobenzodiazepine and motherwort (Leonurus cardiaca L.) dry extract for such applications.
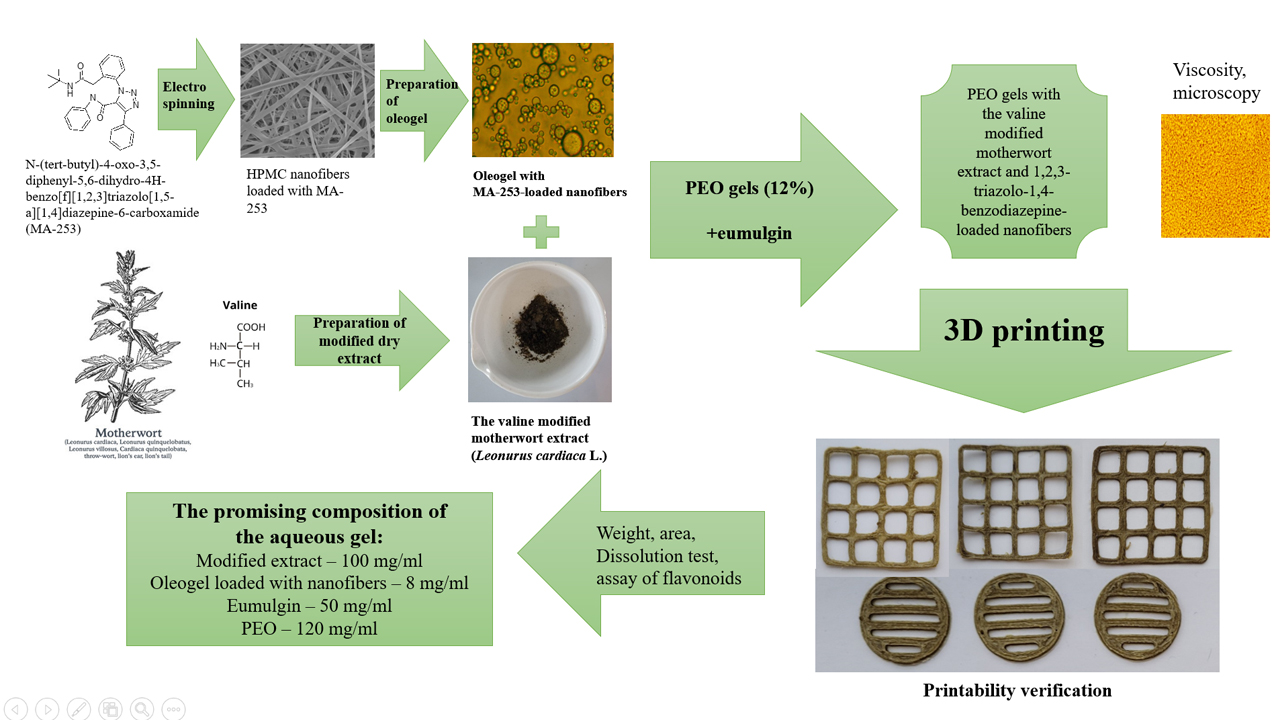
The aim. The aim of this study was to develop aqueous polyethylene oxide (PEO) composite gels loaded with 1,2,3-triazolo-1,4-benzodiazepine nanofibers and a valine-modified motherwort herb dry extract for semi-solid extrusion (SSE) 3D printing. The printability of such gels and the physicochemical properties of the final 3D-printed drug preparations were investigated.
Materials and methods. A new drug substance, 1,2,3-triazolo-1,4-benzodiazepine (MA-253) was synthesized and used to formulate oleogels and electrospun nanofibers for 3D printing. The plant-origin dry extract was prepared from a motherwort tincture and valine. The aqueous PEO gels loaded with a synthetic drug (MA-253) containing nanofibers and a valine-modified motherwort extract were prepared and subsequently used in the SSE 3D printing experiments. The homogeneity, viscosity and 3D printability of composite PEO gels were verified. The phytochemical assay of flavonoids in the 3D-printed drug preparations was conducted with the European pharmacopoeia spectrophotometric method.
Research results. Three experimental gel formulations loaded with 1,2,3-triazolo-1,4-benzodiazepine nanofibers and a valine-modified motherwort dry extract were developed and tested for the SSE 3D printing applications. The present three gels showed good SSE 3D printability without any significant printing flaws. The SSE 3D-printed lattices prepared from the aqueous PEO gels containing 100 mg/ml of motherwort extract showed the most promising 3D printing performance. The 3D-printed drug preparations were entirely dissolved in purified water (22±2 °C) within 20 minutes, thus suggesting their applicability in oral administration.
Conclusions. Novel aqueous PEO gel formulations loaded with nanofibrous 1,2,3-triazolo-1,4-benzodiazepine nanofibers and valine-modified motherwort herb extract are feasible for pharmaceutical SSE 3D printing. The present composite PEO gels enable the preparation of printed oral immediate-release drug delivery systems for new triazolobenzodiazepine derivatives and a drug therapy supportive plant extract
Supporting Agency
- Estonian Research Council grant (PRG1903), CurifyLabs project (VMVFA22189), and the European Union in the MSCA4Ukraine project “Design and development of 3D-printed medicines for bioactive materials of Ukrainian and Estonian medicinal plants origin” [ID number 1232466].
References
- Bandelow, B., Michaelis, S., Wedekind, D. (2017). Treatment of anxiety disorders. Dialogues in Clinical Neuroscience, 19 (2), 93–107. https://doi.org/10.31887/dcns.2017.19.2/bbandelow
- Penninx, B. W., Pine, D. S., Holmes, E. A., Reif, A. (2021). Anxiety disorders. The Lancet, 397 (10277), 914–927. https://doi.org/10.1016/s0140-6736(21)00359-7
- Anxiety disorders (2023). WHO. Available at: https://www.who.int/news-room/fact-sheets/detail/anxiety-disorders Last accessed: 17.01.2024
- Hovenkamp-Hermelink, J. H. M., Jeronimus, B. F., Myroniuk, S., Riese, H., Schoevers, R. A. (2021). Predictors of persistence of anxiety disorders across the lifespan: a systematic review. The Lancet Psychiatry, 8 (5), 428–443. https://doi.org/10.1016/s2215-0366(20)30433-8
- Santomauro, D. F., Mantilla Herrera, A. M., Shadid, J., Zheng, P., Ashbaugh, C., Pigott, D. M. et al. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. The Lancet, 398 (10312), 1700–1712. https://doi.org/10.1016/s0140-6736(21)02143-7
- Vai, B., Mazza, M. G., Delli Colli, C., Foiselle, M., Allen, B., Benedetti, F. et al. (2021). Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: a systematic review and meta-analysis. The Lancet Psychiatry, 8 (9), 797–812. https://doi.org/10.1016/s2215-0366(21)00232-7
- Haustova, O. (2023). Tryvozhno-depresyvni rozlady v umovakh dystresu viiny v Ukraini. Health-ua.com. Available at: https://health-ua.com/article/71710-trivozhnodepresivn-rozladi-vumovah-distresu-vjni-vukran Last accessed: 17.01.2024
- Khan, A., Akram, M., Thiruvengadam, M., Daniyal, M., Zakki, S. A., Munir, N. et al. (2022). Anti-anxiety Properties of Selected Medicinal Plants. Current Pharmaceutical Biotechnology, 23 (8), 1041–1060. https://doi.org/10.2174/1389201022666210122125131
- Van Gool, D., Igodt, P., De Cuyper, H. (1992). Mode of action of the triazolobenzodiazepines in the treatment of panic attacks: a hypothesis. European Neuropsychopharmacology, 2 (4), 433–441. https://doi.org/10.1016/0924-977x(92)90006-t
- Tibrewal, P., Looi, J. C. L., Allison, S., Bastiampillai, T. (2021). Benzodiazepines for the long-term treatment of anxiety disorders? The Lancet, 398 (10295), 119–120. https://doi.org/10.1016/s0140-6736(21)00934-x
- Brett, J., Murnion, B. (2015). Management of benzodiazepine misuse and dependence. Australian Prescriber, 38 (5), 152–155. https://doi.org/10.18773/austprescr.2015.055
- Curado, D. F., de Barros, V. V., Noto, A. R., Opaleye, E. S. (2022). Dependence on hypnotics: a comparative study between chronic users of benzodiazepines and Z-drugs. Brazilian Journal of Psychiatry, 44 (3), 248–256. https://doi.org/10.1590/1516-4446-2020-1651
- Koshovyi, O., Raal, A., Kireyev, I., Tryshchuk, N., Ilina, T., Romanenko, Y. et al. (2021). Phytochemical and Psychotropic Research of Motherwort (Leonurus cardiaca L.) Modified Dry Extracts. Plants, 10 (2), 230. https://doi.org/10.3390/plants10020230
- Rauwald, H., Savtschenko, A., Merten, A., Rusch, C., Appel, K., Kuchta, K. (2015). GABAA Receptor Binding Assays of Standardized Leonurus cardiaca and Leonurus japonicus Extracts as Well as Their Isolated Constituents. Planta Medica, 81 (12/13), 1103–1110. https://doi.org/10.1055/s-0035-1546234
- Qi, J., Hong, Z. Y., Xin, H., Zhu, Y. Z. (2010). Neuroprotective Effects of Leonurine on Ischemia/Reperfusion-Induced Mitochondrial Dysfunctions in Rat Cerebral Cortex. Biological and Pharmaceutical Bulletin, 33 (12), 1958–1964. https://doi.org/10.1248/bpb.33.1958
- Li, Y., Lin, Y., Liu, X., Wang, L., Yu, M., Li, D., Zhu, Y., Du, M. (2019). Leonurine: From Gynecologic Medicine to Pleiotropic Agent. Chinese Journal of Integrative Medicine, 26 (2), 152–160. https://doi.org/10.1007/s11655-019-3453-0
- Koshevoi, O. N. (2011). Amino-acid and monosaccharide compositions of Salvia officinalis leaves. Chemistry of Natural Compounds, 47 (3), 492–493. https://doi.org/10.1007/s10600-011-9976-3
- Romanenko, Y., Koshovyi, O., Ilyina, T., Borodina, N., Melnyk, N. (2019). Standardization parameters of modified extracts from leonurus cardiaca herb. ScienceRise: Pharmaceutical Science, 1 (17), 17–23. https://doi.org/10.15587/2519-4852.2019.157996
- Fernández, S. P., Wasowski, C., Paladini, A. C., Marder, M. (2005). Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. European Journal of Pharmacology, 512 (2-3), 189–198. https://doi.org/10.1016/j.ejphar.2005.02.039
- Tanaka, R., Makino, K., Tabata, H., Oshitari, T., Natsugari, H., Takahashi, H. (2022). Axial chirality and affinity at the GABAA receptor of triazolobenzodiazepines. Bioorganic & Medicinal Chemistry, 64, 116758. https://doi.org/10.1016/j.bmc.2022.116758
- Wojtyniak, K., Szymański, M., Matławska, I. (2012). Leonurus cardiaca L. (Motherwort): A Review of its Phytochemistry and Pharmacology. Phytotherapy Research, 27 (8), 1115–1120. https://doi.org/10.1002/ptr.4850
- File, S. E., Pellow, S. (1985). The effects of triazolobenzodiazepines in two animal tests of anxiety and in the holeboard. British Journal of Pharmacology, 86 (3), 729–735. https://doi.org/10.1111/j.1476-5381.1985.tb08952.x
- Mazur, M. O., Zhelavskyi, O. S., Zviagin, E. M., Shishkina, S. V., Musatov, V. I., Kolosov, M. A. et al. (2021). Effective microwave-assisted approach to 1,2,3-triazolobenzodiazepinones via tandem Ugi reaction/catalyst-free intramolecular azide–alkyne cycloaddition. Beilstein Journal of Organic Chemistry, 17, 678–687. https://doi.org/10.3762/bjoc.17.57
- Botsula, I., Sсhavikin, J., Heinämäki, J., Laidmäe, I., Mazur, M., Raal, A. et al. (2024). Application of nanofiber-based drug delivery systems in improving anxiolytic effect of new 1,2,3-triazolo-1,4-benzodiazepine derivatives. European Journal of Pharmaceutical Sciences, 195, 106712. https://doi.org/10.1016/j.ejps.2024.106712
- Jayakrishna, M., Vijay, M., Khan, B. (2023). An Overview of Extensive Analysis of 3D Printing Applications in the Manufacturing Sector. Journal of Engineering, 2023, 1–23. https://doi.org/10.1155/2023/7465737
- Johannesson, J., Wu, M., Johansson, M., Bergström, C. A. S. (2023). Quality attributes for printable emulsion gels and 3D-printed tablets: Towards production of personalized dosage forms. International Journal of Pharmaceutics, 646, 123413. https://doi.org/10.1016/j.ijpharm.2023.123413
- dos Santos, J., Balbinot, G. de S., Buchner, S., Collares, F. M., Windbergs, M., Deon, M., Beck, R. C. R. (2023). 3D printed matrix solid forms: Can the drug solubility and dose customisation affect their controlled release behaviour? International Journal of Pharmaceutics: X, 5, 100153. https://doi.org/10.1016/j.ijpx.2022.100153
- Cameron, K. O., Beretta, E. E., Chen, Y., Chu-Moyer, M., Fernando, D., Gao, H. et al. (2012). Discovery of new piperidine amide triazolobenzodiazepinones as intestinal-selective CCK1 receptor agonists. Bioorganic & Medicinal Chemistry Letters, 22 (8), 2943–2947. https://doi.org/10.1016/j.bmcl.2012.02.049
- Botsula, I. V., Kireyev, I. V., Koshovyi, O. M., Chebanov, V. A. (2023). The influence of new 1,2,3-triazolo-1,4-benzodiazepine derivatives on the muscle tone of rodents. Current Issues in Pharmacy and Medicine: Science and Practice, 16 (3), 217–222. https://doi.org/10.14739/2409-2932.2023.3.287999
- Koshovyi, O., Heinämäki, J., Laidmäe, I., Topelius, N. S., Grytsyk, A., Raal, A. (2023). Semi-solid extrusion 3D-printing of eucalypt extract-loaded polyethylene oxide gels intended for pharmaceutical applications. Annals of 3D Printed Medicine, 12, 100123. https://doi.org/10.1016/j.stlm.2023.100123
- Azad, M. A., Olawuni, D., Kimbell, G., Badruddoza, A. Z. M., Hossain, Md. S., Sultana, T. (2020). Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics, 12 (2), 124. https://doi.org/10.3390/pharmaceutics12020124
- Anderspuk, H., Viidik, L., Olado, K., Kogermann, K., Juppo, A., Heinämäki, J., Laidmäe, I. (2021). Effects of crosslinking on the physical solid-state and dissolution properties of 3D-printed theophylline tablets. Annals of 3D Printed Medicine, 4, 100031. https://doi.org/10.1016/j.stlm.2021.100031
- Derzhavna Farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1028.
- European Pharmacopoeia (2022) Strasbourg: Council of Europe.
- Ilina, T., Skowrońska, W., Kashpur, N., Granica, S., Bazylko, A., Kovalyova, A. et al. (2020). Immunomodulatory Activity and Phytochemical Profile of Infusions from Cleavers Herb. Molecules, 25 (16), 3721. https://doi.org/10.3390/molecules25163721
- Robakowska, M., Gibson, I., Akkerman, R., Wurm, F. R., Gojzewski, H. (2023). Towards more homogeneous character in 3D printed photopolymers by the addition of nanofillers. Polymer Testing, 129, 108243. https://doi.org/10.1016/j.polymertesting.2023.108243
- Viidik, L., Seera, D., Antikainen, O., Kogermann, K., Heinämäki, J., Laidmäe, I. (2019). 3D-printability of aqueous poly(ethylene oxide) gels. European Polymer Journal, 120, 109206. https://doi.org/10.1016/j.eurpolymj.2019.08.033
- Mohammed, A. A., Algahtani, M. S., Ahmad, M. Z., Ahmad, J. (2021). Optimization of semisolid extrusion (pressure-assisted microsyringe)-based 3D printing process for advanced drug delivery application. Annals of 3D Printed Medicine, 2, 100008. https://doi.org/10.1016/j.stlm.2021.10000
- Wang, N., Shi, H., Yang, S. (2022). 3D printed oral solid dosage form: Modified release and improved solubility. Journal of Controlled Release, 351, 407–431. https://doi.org/10.1016/j.jconrel.2022.09.023
- Macedo, J., Marques, R., Vervaet, C., Pinto, J. F. (2023). Production of Bi-Compartmental Tablets by FDM 3D Printing for the Withdrawal of Diazepam. Pharmaceutics, 15 (2), 538. https://doi.org/10.3390/pharmaceutics15020538
- Wang, M., Li, D., Zang, Z., Sun, X., Tan, H., Si, X. et al. (2021). 3D food printing: Applications of plant-based materials in extrusion-based food printing. Critical Reviews in Food Science and Nutrition, 62 (26), 7184–7198. https://doi.org/10.1080/10408398.2021.1911929
- Raal, A., Jaama, M., Utt, M., Püssa, T., Žvikas, V., Jakštas, V. et al. (2022). The Phytochemical Profile and Anticancer Activity of Anthemis tinctoria and Angelica sylvestris Used in Estonian Ethnomedicine. Plants, 11 (7), 994. https://doi.org/10.3390/plants11070994
- Shang, X., Pan, H., Wang, X., He, H., Li, M. (2014). Leonurus japonicus Houtt.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology, 152 (1), 14–32. https://doi.org/10.1016/j.jep.2013.12.052
- Fierascu, R. C., Fierascu, I., Ortan, A., Fierascu, I. C., Anuta, V., Velescu, B. S. et al. (2019). Leonurus cardiaca L. as a Source of Bioactive Compounds: An Update of the European Medicines Agency Assessment Report (2010). BioMed Research International, 2019, 1–13. https://doi.org/10.1155/2019/4303215
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Iryna Botsula, Igor Kireyev, Oleh Koshovyi, Jyrki Heinämäki, Raal Ain, Maryna Mazur, Valentyn Chebanov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






