A qualitative and quantitative analysis of polyphenolic compounds in five Epilobium spp. with a possible potential to alleviate benign prostatic hyperplasia
DOI:
https://doi.org/10.15587/2519-4852.2024.307139Keywords:
willowherb, oenothein B, myricetin rhamnoside, myricetin glycoside, prostatic hyperplasia, EstoniaAbstract
Benign prostatic hyperplasia (BPH) is a widespread male disease, affecting more than 50 % of men over the age of 60 years. Inhibition of the enzyme 5α-reductase is a common treatment strategy for this condition. Such potential can be found in willow flowers (Epilobium spp.), which are known in folk medicine for treating of prostate ailments, mainly benign prostatitis, hypertrophy and prostatitis. Smallflower hairy willowherb (E. parviflorum), which is rare, is the most recommended for treating BPH.
The aim. The aim of the study was to investigate the qualitative and quantitative content of polyphenols in five Epilobium species (E. adenocaulon Hausskn., E. hirsutum L., E. montanum L., E. parviflorum Schreb. and E. palustre L.) growing in Estonia, to find the most promising species in terms of chemical composition to alleviate BPH.
Materials and Methods. The qualitative and quantitative analyses of polyphenols in herbs of Epilobium spp. were performed using HPLC/MS. All five species were collected from the pond's shore in Pilkuse village (Otepää municipality, Valga county, Estonia) in July 2008.
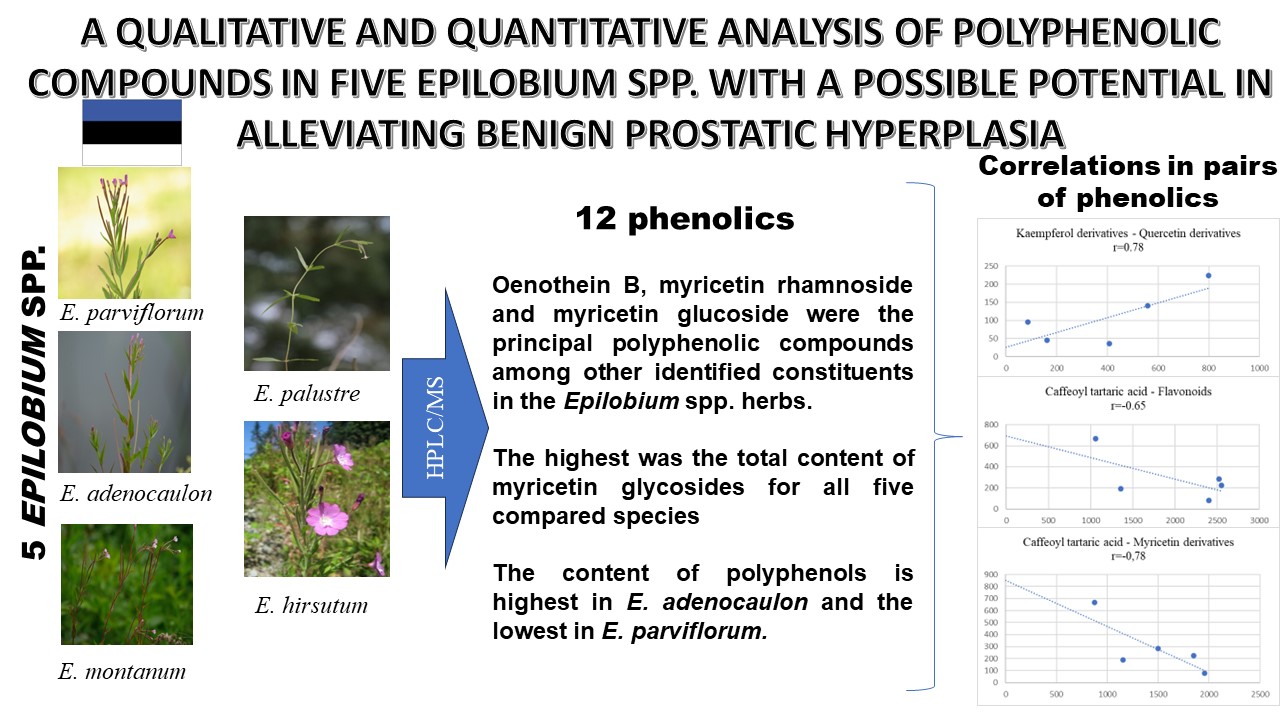
Research results. It was found that 20 % ethanol was optimal for extracting polyphenolic compounds from the herb Epilobium spp. with subsequent UV chromatogram analysis at 350 nm. In the analyzed Epilobium species, 12 polyphenolic compounds were identified. Oenothein B, myricetin rhamnoside and myricetin glucoside were the principal polyphenolic compounds among other identified constituents in the Epilobium spp. herbs. E. montanum had the highest content of oenothein B. The highest was the total content of myricetin glycosides for all five compared species, the total content of quercetin glycosides was slightly lower, and the total content of kaempferol glycosides was the lowest.
Conclusions. The content of polyphenols is highest in E. adenocaulon and the lowest in E. parviflorum. Thus, E. parviflorum does not offer the best therapeutic potential for the to relief of BPH in terms of the quantitative content of polyphenolic compounds
References
- Eesnaare.ee Available at: http://www.eesnaare.ee/index.php?id=359 Last accessed: 21.10.2009
- Thiyagarajan, M. (2002). α-Adrenoceptor Antagonists in the Treatment of Benign Prostate Hyperplasia. Pharmacology, 65 (3), 119–128. https://doi.org/10.1159/000058037
- Thorpe, A., Neal, D. (2003). Benign prostatic hyperplasia. The Lancet, 361 (9366), 1359–1367. https://doi.org/10.1016/s0140-6736(03)13073-5
- Mees.eu Available at: http://www.mees.eu/artikkel/eesnaare-ja-meeste-tervis-1.html Last accessed: 21.10.2009
- Bartsch, G., Rittmaster, R., Klocker, H. (2002). Dihydrotestosterone and the concept of 5α-reductase inhibition in human benign prostatic hyperplasia. World Journal of Urology, 19 (6), 413–425. https://doi.org/10.1007/s00345-002-0248-5
- Ducrey, B., Marston, A., Göhring, S., Hartmann, R., Hostettmann, K. (1997). Inhibition of 5α-Reductase and Aromatase by the Ellagitannins Oenothein A and Oenothein B fromEpilobiumSpecies. Planta Medica, 63 (2), 111–114. https://doi.org/10.1055/s-2006-957624
- Miernik, A., Gratzke, C. (2020). Current Treatment for Benign Prostatic Hyperplasia. Deutsches Ärzteblatt International, 117 (49), 843–854. https://doi.org/10.3238/arztebl.2020.0843
- Rosette, J., Alivizatos, G., Madersbacher, S., Sanz, S. R., Emberton, M., Nordling, J. (2004). Guidelines on Benign Prostatic Hyperplasia. European Association of Urology.
- Oelke, M., Bachmann, A., Descazeaud, A., Emberton, M., Gravas, S., Michel, M. C. et al. (2013). EAU Guidelines on the Treatment and Follow-up of Non-neurogenic Male Lower Urinary Tract Symptoms Including Benign Prostatic Obstruction. European Urology, 64 (1), 118–140. https://doi.org/10.1016/j.eururo.2013.03.004
- Vitalone, A., Bordi, F., Baldazzi, C., Mazzanti, G., Saso, L., Tita, B. (2001). Anti-proliferative effect on a prostatic epithelial cell line (PZ-HPV-7) by Epilobium angustifolium L. Il Farmaco, 56 (5-7), 483–489. https://doi.org/10.1016/s0014-827x(01)01067-9
- Steenkamp, V. (2003). Phytomedicines for the prostate. Fitoterapia, 74 (6), 545–552. https://doi.org/10.1016/s0367-326x(03)00155-2
- Csikós, E., Horváth, A., Ács, K., Papp, N., Balázs, V. L., Dolenc, M. S. et al. (2021). Treatment of Benign Prostatic Hyperplasia by Natural Drugs. Molecules, 26 (23), 7141. https://doi.org/10.3390/molecules26237141
- Hevesi Tóth, B., Blazics, B., Kéry, Á. (2009). Polyphenol composition and antioxidant capacity of Epilobium species. Journal of Pharmaceutical and Biomedical Analysis, 49(1), 26–31. https://doi.org/10.1016/j.jpba.2008.09.047
- Deng, L., Zong, W., Tao, X., Liu, S., Feng, Z., Lin, Y. et al. (2019). Evaluation of the therapeutic effect against benign prostatic hyperplasia and the active constituents from Epilobium angustifolium L. Journal of Ethnopharmacology, 232, 1–10. https://doi.org/10.1016/j.jep.2018.11.045
- Sfriso, R., Claypool, J., Roche, M., Imfeld, D. (2022). 5‐α reductase inhibition by Epilobioum fleischeri extract modulates facial microbiota structure. International Journal of Cosmetic Science, 44 (4), 440–452. https://doi.org/10.1111/ics.12777
- Vitali, F., Fonte, G., Saija, A., Tita, B. (2006). Inhibition of intestinal motility and secretion by extracts of Epilobium spp. in mice. Journal of Ethnopharmacology, 107 (3), 342–348. https://doi.org/10.1016/j.jep.2006.03.025
- Tóth, B. H. (2009). Phytochemical and in vitro biological evaluation of potentially active compounds in Epilobium species. [Thesis of doctoral dissertation; Semmelweis University].
- Lesuisse, D., Berjonneau, J., Ciot, C., Devaux, P., Doucet, B., Gourvest, J. F. et al. (1996). Determination of Oenothein B as the Active 5-α-Reductase-Inhibiting Principle of the Folk Medicine Epilobium parviflorum. Journal of Natural Products, 59 (5), 490–492. https://doi.org/10.1021/np960231c
- Herbal Medicine: Summary for the Public (2016). Willow Herb – Epilobium angustifolium L. and/or Epilobium Parviflorum Schreb., Herba. Available at: https://www.ema.europa.eu/en/medicines/herbal/epilobii-herba Last accessed: 15.12.2022
- Treben, M. (2007). Tervis jumala apteegist. Trak Pen OÜ, 92.
- Epilobium. Available at: http://www.theplantlist.org/1.1/browse/A/Onagraceae/Epilobium/
- Epilobium. Available at: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30000954-2
- Krall, H., Kukk, T., Kull, T., Kuusk, V., Leht, M., Oja, T. et al. (2007). Eesti taimede määraja. Tartu: Eesti Loodusfoto, 448.
- Eichwald, K., Kask, M. (1966). Eesti NSV teaduste akadeemia zooloogia ja botaanika instituut. Eesti taimede määraja.Tallinn: Valgus.
- Kukk, T. (2005). Eesti taimede kukeaabits. Tallinn: Varrak, 396.
- Kukk, T. (1999). Eesti taimestik. Vascular Plant Flora of Estonia. Tartu–Tallinn: Teaduste Akadeemia Kirjastus, 464.
- Lundvall, C-F., Björkman, G. (2007). Põhjamaa õistaimed. Umbes 370 tavalist looduses kasvavat taime. Tallinn: Varrak, 348.
- Vlase, A.-M., Toiu, A., Gligor, O., Muntean, D., Casian, T., Vlase, L. et al. (2024). Investigation of Epilobium hirsutum L. Optimized Extract’s Anti-Inflammatory and Antitumor Potential. Plants, 13, 198. https://doi.org/10.3390/plants13020198
- Kyriakou, S., Tragkola, V., Paraskevaidis, I., Plioukas, M., Trafalis, D. T., Franco, R. et al. (2023). Chemical Characterization and Biological Evaluation of Epilobium parviflorum Extracts in an In Vitro Model of Human Malignant Melanoma. Plants, 12 (8), 1590. https://doi.org/10.3390/plants12081590
- Granica, S., Piwowarski, J. P., Czerwińska, M. E., Kiss, A. K. (2014). Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. Journal of Ethnopharmacology, 156, 316–346. https://doi.org/10.1016/j.jep.2014.08.036
- Abbasi Karin, Sh., Karimzadeh, G., Mohammadi Bazargani, M. (2023). Interspecific morphological and phytochemical variation in the willow herb (Epilobium spp.) medicinal plant. Journal of Plant Physiology and Breeding, 13 (2), 15–27.
- Ivanauskas, L., Uminska, K., Gudžinskas, Z., Heinrich, M., Georgiyants, V., Kozurak, A., Mykhailenko, O. (2023). Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine. Plants, 13 (1), 120. https://doi.org/10.3390/plants13010120
- Comalada, M., Camuesco, D., Sierra, S., Ballester, I., Xaus, J., Gálvez, J., Zarzuelo, A. (2005). In vivo quercitrin anti‐inflammatory effect involves release of quercetin, which inhibits inflammation through down‐regulation of the NF‐κB pathway. European Journal of Immunology, 35 (2), 584–592. https://doi.org/10.1002/eji.200425778
- Ong, K. C., Khoo, H.-E. (1997). Biological effects of myricetin. General Pharmacology: The Vascular System, 29 (2), 121–126. https://doi.org/10.1016/s0306-3623(96)00421-1
- Vitalone, A., Allkanjari, O. (2018). Epilobium spp: Pharmacology and Phytochemistry. Phytotherapy Research, 32 (7), 1229–1240. https://doi.org/10.1002/ptr.6072
- Jariene, E., Lasinskas, M., Danilcenko, H., Vaitkeviciene, N., Slepetiene, A., Najman, K., Hallmann, E. (2020). Polyphenols, Antioxidant Activity and Volatile Compounds in Fermented Leaves of Medicinal Plant Rosebay Willowherb (Chamerion angustifolium (L.) Holub). Plants, 9 (12), 1683. https://doi.org/10.3390/plants9121683
- Constantin, D., Coste, A., Mircea, T.; Chandra, S., Lata, H., Varma, A. (Eds.) (2013). Epilobium sp. (Willow Herb): Micropropagation and production of secondary metabolites. Biotechnology for medicinal plants. Berlin: Springer, 149–170. https://doi.org/10.1007/978-3-642-29974-2_6
- Vlase, A.-M., Toiu, A., Tomuță, I., Vlase, L., Muntean, D., Casian, T. et al. (2022). Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants, 12 (1), 91. https://doi.org/10.3390/antiox12010091
- Agnieszka, G., Mariola, D., Anna, P., Piotr, K., Natalia, W., Aneta, S. et al. (2018). Qualitative and quantitative analyses of bioactive compounds from ex vitro Chamaenerion angustifolium (L.) (Epilobium augustifolium) herb in different harvest times. Industrial Crops and Products, 123, 208–220. https://doi.org/10.1016/j.indcrop.2018.06.010
- Gonthier, M.-P., Remesy, C., Scalbert, A., Cheynier, V., Souquet, J.-M., Poutanen, K., Aura, A.-M. (2006). Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomedicine & Pharmacotherapy, 60 (9), 536–576. https://doi.org/10.1016/j.biopha.2006.07.084
- Schepetkin, I. A., Kirpotina, L. N., Jakiw, L., Khlebnikov, A. I., Blaskovich, C. L., Jutila, M. A., Quinn, M. T. (2009). Immunomodulatory Activity of Oenothein B Isolated from Epilobium angustifolium. The Journal of Immunology, 183 (10), 6754–6766. https://doi.org/10.4049/jimmunol.0901827
- Ak, G., Zengin, G., Mahomoodally, M. F., Llorent-Martínez, E., Orlando, G., Chiavaroli, A. et al. (2021). Shedding Light into the Connection between Chemical Components and Biological Effects of Extracts from Epilobium hirsutum: Is It a Potent Source of Bioactive Agents from Natural Treasure? Antioxidants, 10 (9), 1389. https://doi.org/10.3390/antiox10091389
- Dreger, M., Adamczak, A., Seidler-Łożykowska, K., Wielgus, K. (2020). Pharmacological properties of fireweed (Epilobium angustifolium L.) and bioavailability of ellagitannins. A review. Herba Polonica, 66 (1), 52–64. https://doi.org/10.2478/hepo-2020-0001
- Sultana, B., Anwar, F. (2008). Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chemistry, 108 (3), 879–884. https://doi.org/10.1016/j.foodchem.2007.11.053
- Esposito, C., Santarcangelo, C., Masselli, R., Buonomo, G., Nicotra, G., Insolia, V. et al. (2021). Epilobium angustifolium L. extract with high content in oenothein B on benign prostatic hyperplasia: A monocentric, randomized, double-blind, placebo-controlled clinical trial. Biomedicine & Pharmacotherapy, 138, 111414. https://doi.org/10.1016/j.biopha.2021.111414
- Miyamoto, K., Nomura, M., Sasakura, M., Matsui, E., Koshiura, R., Murayama, T. et al. (1993). Antitumor Activity of Oenothein B, a Unique Macrocyclic Ellagitannin. Japanese Journal of Cancer Research, 84 (1), 99–103. https://doi.org/10.1111/j.1349-7006.1993.tb02790.x
- Kiss, A. K., Bazylko, A., Filipek, A., Granica, S., Jaszewska, E., Kiarszys, U. et al. (2011). Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine, 18 (7), 557–560. https://doi.org/10.1016/j.phymed.2010.10.016
- Deng, L.-Q., Zhou, S.-Y., Mao, J.-X., Liu, S., Lan, X.-Z., Liao, Z.-H., Chen, M. (2017). HPLC-ESI-MS/MS analysis of phenolics and in vitro antioxidant activity of Epilobium angustifolium L. Natural Product Research, 32 (12), 1432–1435. https://doi.org/10.1080/14786419.2017.1344659
- Jürgenson, S., Matto, V., Raal, A. (2012). Vegetational variation of phenolic compounds inEpilobium angustifolium. Natural Product Research, 26 (20), 1951–1953. https://doi.org/10.1080/14786419.2011.643310
- Remmel, I., Vares, L., Toom, L., Matto, V., Raal, A. (2012). Phenolic Compounds in FiveEpilobiumSpecies Collected from Estonia. Natural Product Communications, 7 (10), 1323–1327. https://doi.org/10.1177/1934578x1200701017
- Dürüst, N., Dürüst, Ya., İkinci, N., Banko, S., Zafer, Hoşgün E., Bozan, B. (2023). HPLC determination of polyphenols of the flowers of Digitalis lamarckii, Xeranthemum annuum, Epilobium hirsutum and Silene compacta from Bolu (Turkey). Journal of Medicinal Plants Research, 17 (5), 164–179. doi: 10.5897/jmpr2022.7282
- Lin, P., Wang, X., Zhou, N., Wu, Y., Wang, Z., Wu, L. et al. (2021). Chemical characterization of the anti-inflammatory activity fraction of Epilobium angustifolium. European Food Research and Technology, 248 (1), 35–44. https://doi.org/10.1007/s00217-021-03831-w
- Yoshida, T., Yoshimura, M., Amakura, Y. (2018). Chemical and Biological Significance of Oenothein B and Related Ellagitannin Oligomers with Macrocyclic Structure. Molecules, 23 (3), 552. https://doi.org/10.3390/molecules23030552
- Vitalone, A., Guizzetti, M., Costa, L. G., Tita, B. (2003). Extracts of various species of Epilobium inhibit proliferation of human prostate cells. Journal of Pharmacy and Pharmacology, 55 (5), 683–690. https://doi.org/10.1211/002235703765344603
- Vitalone, A., McColl, J., Thome, D., Costa, L. G., Tita, B. (2003). Characterization of the Effect of Epilobium Extracts on Human Cell Proliferation. Pharmacology, 69 (2), 79–87. https://doi.org/10.1159/000072360
- Hiipakka, R. A., Zhang, H.-Z., Dai, W., Dai, Q., Liao, S. (2002). Structure–activity relationships for inhibition of human 5α-reductases by polyphenols. Biochemical Pharmacology, 63 (6), 1165–1176. https://doi.org/10.1016/s0006-2952(02)00848-1
- Marzullo, L., Ochkur, O., Orlandini, S., Renai, L., Gotti, R., Koshovyi, O. et al. (2022). Quality by Design in optimizing the extraction of (poly)phenolic compounds from Vaccinium myrtillus berries. Journal of Chromatography A, 1677, 463329. https://doi.org/10.1016/j.chroma.2022.463329
- Taheri, Y., Suleria, H. A. R., Martins, N., Sytar, O., Beyatli, A., Yeskaliyeva, B. et al. (2020). Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC Complementary Medicine and Therapies, 20 (1). https://doi.org/10.1186/s12906-020-03033-z
- Vafadar, A., Shabaninejad, Z., Movahedpour, A., Fallahi, F., Taghavipour, M., Ghasemi, Y. et al. (2020). Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell & Bioscience, 10 (1). https://doi.org/10.1186/s13578-020-00397-0
- Huzio, N., Grytsyk, A., Raal, A., Grytsyk, L., Koshovyi, O. (2022). Phytochemical and Pharmacological Research in Agrimonia eupatoria L. Herb Extract with Anti-Inflammatory and Hepatoprotective Properties. Plants, 11 (18), 2371. https://doi.org/10.3390/plants11182371
- Bai, F., Wang, Y., Zhang, S., Wang, Y., Zhang, J., Cao, J., Sun, L. (2020). Caffeoyl substitution changes the inhibition mode of tartaric acid against α-amylase: Analysis of the enzyme inhibition by four caffeic and tartaric acid derivates. LWT, 133, 109942. https://doi.org/10.1016/j.lwt.2020.109942
- Sidoryk, K., Jaromin, A., Filipczak, N., Cmoch, P., Cybulski, M. (2018). Synthesis and Antioxidant Activity of Caffeic Acid Derivatives. Molecules, 23 (9), 2199. https://doi.org/10.3390/molecules23092199
- Walker, K. J. (2007). The last thirty five years: recent changes in the flora of the British Isles. New Journal of Botany, 26, 291–302.
- Naikoo, M. I., Dar, M. I., Raghib, F., Jaleel, H., Ahmad, B., Raina, A. et al. (2019). Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance. Plant Signaling Molecules. Elsevier, 157–168. https://doi.org/10.1016/b978-0-12-816451-8.00009-5
- Bleeker, W., Schmitz, U., Ristow, M. (2007). Interspecific hybridisation between alien and native plant species in Germany and its consequences for native biodiversity. Biological Conservation, 137 (2), 248–253. https://doi.org/10.1016/j.biocon.2007.02.004
- Vlasova, I., Gontova, T., Grytsyk, L., Zhumashova, G., Sayakova, G., Boshkayeva, A. et al. (2022). Determination of standardization parameters of Oxycoccus macrocarpus (Ait.) Pursh and Oxycoccus palustris Pers. Leaves. ScienceRise: Pharmaceutical Science, 3 (37), 48–57. https://doi.org/10.15587/2519-4852.2022.260352
- Wittenberg, R. (Ed.) (2005). An inventory of alien species and their threat to biodiversity and economy in Switzerland. CABI Bioscience Switzerland Centre report to the Swiss Agency for Environment, Forests and Landscape SAEFL, 356.
- Krajšek, S.S., Jogan, N. (2004). Epilobium ciliatum Raf., a new plant invader in Slovenia and Croatia. Acta Botanica Croatica, 63 (1), 49–58.
- Rakhmetov, D. B. (2017). Secondary metabolism in the adaptation of introduced plants in Ukraine. Stress factors and secondary metabolites. Kyiv, 14–15.
- Badria, F. A. (Ed.) (2022). Phenolic Compounds: Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications. Biochemistry. IntechOpen, 452. https://doi.org/10.5772/intechopen.94825
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Raal Ain, Kristiina Kuiv, Тetiana Ilina, Alla Kovaleva, Yuliia Avidzba, Oleh Koshovyi, Püssa Tõnu

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






