Study of the influence of the extract of pipsissewa on cell cultures
DOI:
https://doi.org/10.15587/2519-4852.2024.307291Keywords:
Chimaphila umbellata (L.), cell culture L929, proliferation, adhesion, cell migrationAbstract
The development of new diuretics of plant origin is an actual direction. Chimaphila umbellata (L.) is a perennial herb with diuretic, astringent, analgesic and other effects; and it can treat various conditions such as edema, dropsy, etc. Pipsissewa herb helps the removal of nitrogenous and chloride salts from the body due to the content of arbutin glycoside, tannins (up to 5 %).
The aim. Evaluation of the effect of pipsissewa extract on L929 cell culture.
Materials and methods. Cell line L929 (fibroblasts of mouse adipose tissue) was obtained in the low-temperature bank of the Institute of Problems of Cryobiology and Cryomedicine of the National Academy of Sciences of Ukraine. Cells were cultured in DMEM medium (Bio West, France) enriched with 10 % FBS (Lonza, Germany) with 1 % antibiotic-antimycotic (Bio West, France), in a CO2 incubator (Thermo Fisher Scientific, USA) at 37 ºC in an atmosphere with 5 % CO2.
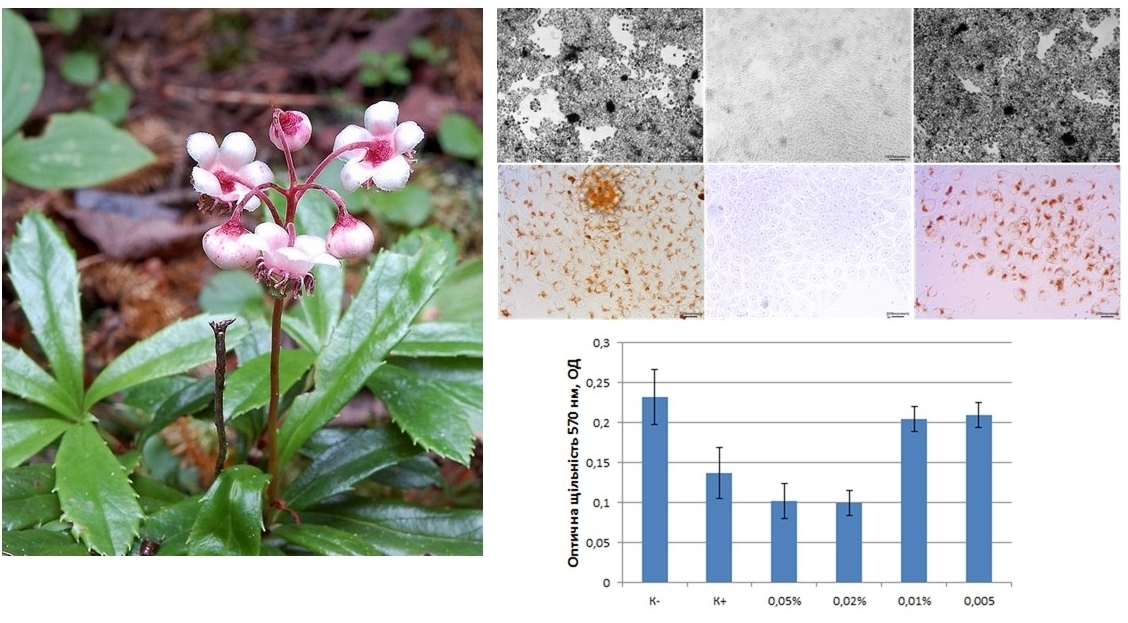
Determination of the minimum toxic concentration at which the cells remained alive was evaluated by morphological features (shape, monolayer integrity, adhesion to plastic). The study of the effect of pipsissewa extract on various cell functions was determined by the following methods: the ability to preserve morphological integrity - by the phase-contrast microscopy method, energy exchange - by the MTT test method, pinocytotic function - by the neutral red absorption method, migratory function - by the scratch test method, proliferative activity - by the doubling calculation method population
Results. It is proposed to use concentrations of 0.05, 0.02, 0.01, 0.005 % of pipsissewa extract for further research. After carrying out the MTT reaction, the transition of MTT to formazan was confirmed microscopically in the negative control (native cells), at PE concentrations of 0.01 % and below, and the absence of a reaction in the positive control (cells killed by ethanol) at PE concentrations above 0.02 %. When recording the parameters of the NP absorption reaction, it was determined that PE at a concentration of 0.02 % and higher sharply suppresses pinocytotic activity, despite the partial preservation of cell adhesion, reducing the concentration by two times no longer affects mitochondria. A concentration of 0.01 % reduces proliferative activity, and at a concentration of 0.005 %, no difference with the control values was found.
Conclusions. When studying the assessment of the effect of pipsisewa extract on L929 cell culture, a toxic effect on these cells was established when added to the culture medium at a concentration above 0.01 %. The toxic effect had a threshold effect. Migratory and proliferative functions were the most sensitive, energy, pinocytosis and preservation of morphological integrity of cells were less sensitive
References
- Roush, G. C., Sica, D. A. (2016). Diuretics for Hypertension: A Review and Update. American Journal of Hypertension, 29 (10), 1130–1137. https://doi.org/10.1093/ajh/hpw030
- Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G. F., Coats, A. J. S. et al. (2016). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal, 37 (27), 2129–2200. https://doi.org/10.1093/eurheartj/ehw128
- Minutolo, R., De Nicola, L., Mallamaci, F., Zoccali, C. (2022). Thiazide diuretics are back in CKD: the case of chlorthalidone. Clinical Kidney Journal, 16 (1), 41–51. https://doi.org/10.1093/ckj/sfac198
- Chronic heart failure in adults: diagnosis and management (2018). NICE. Available at: https://www.nice.org.uk/guidance/ng106/resources/chronic-heart-failure-in-adults-diagnosis-and-management-pdf-66141541311685
- Vandal, J., Abou-Zaid, M. M., Ferroni, G., Leduc, L. G. (2015). Antimicrobial activity of natural products from the flora of Northern Ontario, Canada. Pharmaceutical Biology, 53 (6), 800–806. https://doi.org/10.3109/13880209.2014.942867
- Ahmadi, E. S., Tajbakhsh, A., Iranshahy, M., Asili, J., Kretschmer, N., Shakeri, A., Sahebkar, A. (2020). Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity. Mini-Reviews in Medicinal Chemistry, 20 (19), 2019–2035. https://doi.org/10.2174/1389557520666200818212020
- Safarzadeh, E., Shotorbani, S. S., Baradaran, B. (2014). Herbal medicine as inducers of apoptosis in cancer treatment. Advanced Pharmaceutical Bulletin, 4 (Suppl 1), 421–427.
- Sherstiuk, M. (2017). The analysis of vitality structure of Chimaphila Umbellata (L.) W. Barton CENOpopulations in forest phytocenoses of the Novgorod-Sivers’k Polissia. ScienceRise: Biological Science, 1 (4), 40–45. https://doi.org/10.15587/2519-8025.2017.94019
- Dong, F., Liu, T., Jin, H., Wang, W. (2018). Chimaphilin inhibits human osteosarcoma cell invasion and metastasis through suppressing the TGF-β1-induced epithelial-to-mesenchymal transition markers via PI-3K/Akt, ERK1/2, and Smad signaling pathways. Canadian Journal of Physiology and Pharmacology, 96 (1), 1–7. https://doi.org/10.1139/cjpp-2016-0522
- Hrodzinskyi, A. M. (1992). Likarski roslyny. Kyiv: Vydavnytstvo «Ukrainska Entsyklopediia» im. M. P. Bazhana, Ukrainskyi vyrobnycho-komertsiinyi tsentr «Olimp», 544.
- Podpletnyaia, E. A., Khomiak, N. V., Sokolova, E. V., Kaydash, S. P., Khomiak, E. V. (2017). Fitoterapevtychni likarski zasoby z nefroprotektornoiu aktyvnistiu (ohliad). Medical perspectives, 22 (1), 10–19.
- Jütte, R., Riley, D. (2005). A review of the use and role of low potencies in homeopathy. Complementary Therapies in Medicine, 13 (4), 291–296. https://doi.org/10.1016/j.ctim.2005.10.003
- Ali, U., Khan, M. M., Khan, N., Haya, R. tul, Asghar, M. U., Abbasi, B. H. (2023). Chimaphila umbellata; a biotechnological perspective on the coming-of-age prince’s pine. Phytochemistry Reviews, 23 (1), 229–244. https://doi.org/10.1007/s11101-023-09880-1
- Culenova, M., Nicodemou, A., Novakova, Z. V., Debreova, M., Smolinská, V., Bernatova, S. et al. (2021). Isolation, Culture and Comprehensive Characterization of Biological Properties of Human Urine-Derived Stem Cells. International Journal of Molecular Sciences, 22 (22), 12503. https://doi.org/10.3390/ijms222212503
- Yu, Y., Hu, D., Liu, J., Wu, C., Sun, Y., Lang, M. et al. (2024). Constituents of Chimaphila japonica and Their Diuretic Activity. Molecules, 29 (5), 1092. https://doi.org/10.3390/molecules29051092
- Hitomi, J., Christofferson, D. E., Ng, A., Yao, J., Degterev, A., Xavier, R. J., Yuan, J. (2008). Identification of a Molecular Signaling Network that Regulates a Cellular Necrotic Cell Death Pathway. Cell, 135 (7), 1311–1323. https://doi.org/10.1016/j.cell.2008.10.044
- Bozhok, G. A., Moisieiev, A. I., Gorina, O. L., Bondarenko, T. P. (2019). Morphofunctional features of fibroblasts line L929 in 3D-culture. Fiziolohichnyĭ Zhurnal, 65 (3), 34–40. https://doi.org/10.15407/fz65.03.034
- Priyadarshini, P., Samuel, S., Kurkalli, B. G., Kumar, C., Kumar, B. M., Shetty, N. et al. (2021). In vitro Comparison of Adipogenic Differentiation in Human Adipose-Derived Stem Cells Cultured with Collagen Gel and Platelet-Rich Fibrin. Indian Journal of Plastic Surgery, 54 (3), 278–283. https://doi.org/10.1055/s-0041-1733810
- Hulkower, K. I., Herber, R. L. (2011). Cell Migration and Invasion Assays as Tools for Drug Discovery. Pharmaceutics, 3 (1), 107–124. https://doi.org/10.3390/pharmaceutics3010107
- Gomez Perez, M., Fourcade, L., Mateescu, M. A., Paquin, J. (2017). Neutral Red versus MTT assay of cell viability in the presence of copper compounds. Analytical Biochemistry, 535, 43–46. https://doi.org/10.1016/j.ab.2017.07.027
- Kumar, P., Nagarajan, A., Uchil, P. D. (2018). Analysis of Cell Viability by the MTT Assay. Cold Spring Harbor Protocols, 2018 (6), pdb.prot095505. https://doi.org/10.1101/pdb.prot095505
- Repetto, G., del Peso, A., Zurita, J. L. (2008). Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nature Protocols, 3 (7), 1125–1131. https://doi.org/10.1038/nprot.2008.75
- Das, N., Samantaray, S., Ghosh, C., Kushwaha, K., Sircar, D., Roy, P. (2022). Chimaphila umbellata extract exerts anti-proliferative effect on human breast cancer cells via RIP1K/RIP3K-mediated necroptosis. Phytomedicine Plus, 2 (1), 100159. https://doi.org/10.1016/j.phyplu.2021.100159
- Preethi, K., Ellanghiyil, S., Kuttan, G., Kuttan, R. (2011). Induction of Apoptosis of Tumor Cells by Some Potentiated Homeopathic Drugs. Integrative Cancer Therapies, 11 (2), 172–182. https://doi.org/10.1177/1534735411400310
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Oleksiy Kovregin, Volodymyr Prokopiuk, Dmytro Lytkin, Inna Vladymyrova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






