Synthesis and nootropic activity prediction of some 4-(aminomethyl)-1-benzylpyrrolidin-2-one derivatives structurally related with nebracetam
DOI:
https://doi.org/10.15587/2519-4852.2024.310731Keywords:
synthesis, 4-(aminomethyl)-1-benzylpyrrolidin-2-one, molecular docking, acetylcholine receptors, nootropic activityAbstract
The aim. Search for new biologically active substances with improved nootropic parameters among analogues of 4-(aminomethyl)-1-benzylpyrrolidine-2-one (Nebracetam).
Materials and methods. The required reagents were purified using standard techniques. The elemental analysis was performed on a "Hewlett Packard" automatic analyzer M-180 company. 1H NMR spectra were recorded on Varian Gemini 400 MHz spectrometer in DMSO-d6 as a solvent. LC/MS spectra were recorded with a PE SCIEX API 150EX liquid chromatograph equipped. The Autodock 4.2 software package was used for molecular docking. The active centers of the peptides (PDB ID: 5CXV, 6PV7) was used as the biologycal targets.
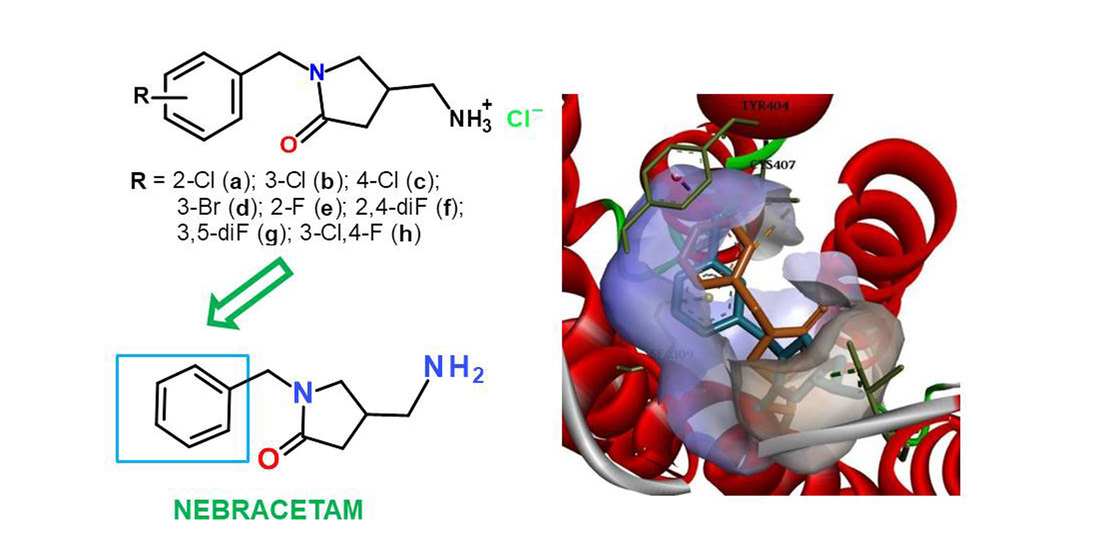
Results and discussion. Basic and alternative methods (1 and 2) of obtaining were used to synthesise target analogues of 4-(aminomethyl)-1-R-benzylpyrrolidine-2-one. As a result of synthetic studies, an optimized method with an alternative method has been proposed. The advantages include reducing the duration and number of synthesis stages and avoiding the use of sodium azide, a highly toxic and hazardous substance. Molecular docking of the synthesized compounds at well-documented acetylcholine receptor sites indicates that all tested molecules will contribute to the manifestation of nootropic activity to varying degrees through cholinergic neurotransmission mechanisms. This is evidenced by the calculated docking values in relation to the muscarinic target. According to the docking results, it was found that depending on the enantiomeric configuration, the molecules formed stable complexes with the target and had characteristic binding modes both in the orthosteric site and in the extracellular vestibule (site of positive allosteric modulation of mAChR). It indicates the prospects of modifying the "nebracetam scaffold" at the phenyl fragment with halogen substituents.
Conclusions. An effective method for synthesising analogues of 4-(aminomethyl)-1-R-benzylpyrrolidin-2-ones has been developed. The molecular docking revealed potential mechanisms of nootropic action of the synthesized derivatives as potential agonists and positive allosteric modulators of the muscarinic receptor
References
- Li Petri, G., Raimondi, M. V., Spanò, V., Holl, R., Barraja, P., Montalbano, A. (2021). Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Topics in Current Chemistry, 379 (5). https://doi.org/10.1007/s41061-021-00347-5
- Saiz Garcia, H., Montes Reula, L., Portilla Fernandez, A., Pereira Sanchez, V., Olmo Lopez, N., Mancha Heredero, E. et al. (2017). Nootropics: Emergents drugs associated with new clinical challenges. European Psychiatry, 41 (S1), s877–s878. https://doi.org/10.1016/j.eurpsy.2017.01.1769
- Alfaro-Rodríguez, A., Cortes-Altamirano, J., Olmos-Hernández, A., Bonilla-Jaime, H., Bandala, C., González-Maciel, A. (2016). Levetiracetam as an antiepileptic, neuroprotective, and hyperalgesic drug. Neurology India, 64 (6), 1266–1275. https://doi.org/10.4103/0028-3886.193801
- Urakami, K., Shimomura, T., Ohshima, T., Okada, A., Adachi, Y., Takahashi, K. et al. (1993). Clinical Effect of WEB 1881 (Nebracetam Fumarate) on Patients with Dementia of the Alzheimer Type and Study of Its Clinical Pharmacology. Clinical Neuropharmacology, 16 (4), 347–358. https://doi.org/10.1097/00002826-199308000-00007
- Kitamura, Y., Kaneda, T., Nomura, Y. (1991). Effects of nebracetam (WEB 1881 FU), a novel nootropic, as a M1-muscarinic agonist. The Japanese Journal of Pharmacology, 55 (1), 177–180. https://doi.org/10.1254/jjp.55.177
- Sahakian, B. J., Morein-Zamir, S. (2015). Pharmacological cognitive enhancement: treatment of neuropsychiatric disorders and lifestyle use by healthy people. The Lancet Psychiatry, 2 (4), 357–362. https://doi.org/10.1016/s2215-0366(15)00004-8
- Levin, E. D., Sledge, D., Roach, S., Petro, A., Donerly, S., Linney, E. (2011). Persistent behavioral impairment caused by embryonic methylphenidate exposure in zebrafish. Neurotoxicology and Teratology, 33 (6), 668–673. https://doi.org/10.1016/j.ntt.2011.06.004
- Battleday, R. M., Brem, A.-K. (2015). Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. European Neuropsychopharmacology, 25 (11), 1865–1881. https://doi.org/10.1016/j.euroneuro.2015.07.028
- Vyas, S., Kothari, S. L., Kachhwaha, S. (2019). Nootropic medicinal plants: Therapeutic alternatives for Alzheimer’s disease. Journal of Herbal Medicine, 17-18, 100291. https://doi.org/10.1016/j.hermed.2019.100291
- Richter, N., Allendorf, I., Onur, O. A., Kracht, L., Dietlein, M., Tittgemeyer, M. et al. (2014). The integrity of the cholinergic system determines memory performance in healthy elderly. NeuroImage, 100, 481–488. https://doi.org/10.1016/j.neuroimage.2014.06.031
- Malykh, A. G., Sadaie, M. R. (2010). Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs, 70 (3), 287–312. https://doi.org/10.2165/11319230-000000000-00000
- Uniyal, A., Singh, R., Akhtar, A., Bansal, Y., Kuhad, A., Sah, S. P. (2019). Co-treatment of piracetam with risperidone rescued extinction deficits in experimental paradigms of post-traumatic stress disorder by restoring the physiological alterations in cortex and hippocampus. Pharmacology Biochemistry and Behavior, 185, 172763. https://doi.org/10.1016/j.pbb.2019.172763
- Grossman, L., Stewart, A., Gaikwad, S., Utterback, E., Wu, N., DiLeo, J. et al. (2011). Effects of piracetam on behavior and memory in adult zebrafish. Brain Research Bulletin, 85(1–2), 58–63. https://doi.org/10.1016/j.brainresbull.2011.02.008
- Krintel, C., Harpsøe, K., Zachariassen, L. G., Peters, D., Frydenvang, K., Pickering, D. S. et al. (2013). Structural analysis of the positive AMPA receptor modulators CX516 and Me-CX516 in complex with the GluA2 ligand-binding domain. Acta Crystallographica Section D Biological Crystallography, 69 (9), 1645–1652. https://doi.org/10.1107/s0907444913011839
- Pugsley, T. A., Shih, Y., Coughenour, L., Stewart, S. F. (1983). Some neurochemical properties of pramiracetam (CI‐879), a new cognition‐enhancing agent. Drug Development Research, 3 (5), 407–420. https://doi.org/10.1002/ddr.430030503
- Nakashima, M. N., Kataoka, Y., Yamashita, K., Kohzuma, M., Ichikawa, M., Niwa, M. et al. (1995). Histological Evidence for Neuroprotective Action of Nebracetam on Ischemic Neuronal Injury in the Hippocampus of Stroke-Prone Spontaneously Hypertensive Rats. Japanese Journal of Pharmacology, 67 (1), 91–94. https://doi.org/10.1254/jjp.67.91
- Iwasaki, K., Matsumoto, Y., Fujiwara, M. (1992). Effect of Nebracetam on the Disruption of Spatial Cognition in Rats. The Japanese Journal of Pharmacology, 58 (2), 117–126. https://doi.org/10.1254/jjp.58.117
- Semenets, A. P., Suleiman, M. M., Fedosov, A. I., Shtrygol, S. Y., Havrylov, I. O., Mishchenko, M. V. et al. (2022). Synthesis, docking, and biological evaluation of novel 1-benzyl-4-(4-(R)-5-sulfonylidene-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidin-2-ones as potential nootropic agents. European Journal of Medicinal Chemistry, 244, 114823. https://doi.org/10.1016/j.ejmech.2022.114823
- Semenets, A., Suleiman, M., Georgiyants, V., Kovalenko, S., Kobzar, N., Grinevich, L. et al. (2020). Theoretical justification of a purposeful search of potential neurotropic drugs. ScienceRise: Pharmaceutical Science, 4 (26), 4–17. https://doi.org/10.15587/2519-4852.2020.210042
- Yamashita, S., Mase, N., Takabe, K. (2008). Chemoenzymatic total synthesis and determination of the absolute configuration of (S)-nebracetam. Tetrahedron: Asymmetry, 19 (18), 2115–2118. https://doi.org/10.1016/j.tetasy.2008.09.004
- Gharpure, A., Teng, J., Zhuang, Y., Noviello, C. M., Walsh, R. M., Cabuco, R. et al. (2019). Agonist Selectivity and Ion Permeation in the α3β4 Ganglionic Nicotinic Receptor. Neuron, 104 (3), 501-511.e6. https://doi.org/10.1016/j.neuron.2019.07.030
- Grady, S. R., Moretti, M., Zoli, M., Marks, M. J., Zanardi, A., Pucci, L. et al (2009). Rodent Habenulo–Interpeduncular Pathway Expresses a Large Variety of Uncommon nAChR Subtypes, But Only the α3β4 and α3β3β4 Subtypes Mediate Acetylcholine Release. The Journal of Neuroscience, 29 (7), 2272–2282. https://doi.org/10.1523/jneurosci.5121-08.2009
- Albuquerque, E. X., Pereira, E. F. R., Alkondon, M., Rogers, S. W. (2009). Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiological Reviews, 89 (1), 73–120. https://doi.org/10.1152/physrev.00015.2008
- Changeux, J.-P. (2018). The nicotinic acetylcholine receptor: a typical ‘allosteric machine.’ Philosophical Transactions of the Royal Society B: Biological Sciences, 373 (1749), 20170174. https://doi.org/10.1098/rstb.2017.0174
- Park, Y.-S., Kim, J., Kim, S.-H., Moon, Y.-J., Kwon, H.-M., Park, H.-S. et al. (2019). Comparison of recovery profiles in patients with Parkinson’s disease for 2 types of neuromuscular blockade reversal agent following deep brain stimulator implantation. Medicine, 98 (52), e18406. https://doi.org/10.1097/md.0000000000018406
- Thal, D. M., Sun, B., Feng, D., Nawaratne, V., Leach, K., Felder, C. C. et al. (2016). Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature, 531 (7594), 335–340. https://doi.org/10.1038/nature17188
- Kruse, A. C., Kobilka, B. K., Gautam, D., Sexton, P. M., Christopoulos, A., Wess, J. (2014). Muscarinic acetylcholine receptors: novel opportunities for drug development. Nature Reviews Drug Discovery, 13 (7), 549–560. https://doi.org/10.1038/nrd4295
- Changeux, J.-P. (2013). The concept of allosteric modulation: an overview. Drug Discovery Today: Technologies, 10 (2), e223–e228. https://doi.org/10.1016/j.ddtec.2012.07.007
- Dhama, N., Sucheta, Kumar, A., Verma, V., Kumar, S. (2021). A Review on Synthesis and Pharmacological Activities of Piracetam and its Derivatives. Asian Journal of Chemistry, 34 (1), 1–8. https://doi.org/10.14233/ajchem.2022.23357
- Jean, L., Baglin, I., Rouden, J., Maddaluno, J., Lasne, M.-C. (2001). A convenient route to 1-benzyl 3-aminopyrrolidine and 3-aminopiperidine. Tetrahedron Letters, 42 (33), 5645–5649. https://doi.org/10.1016/s0040-4039(01)00985-6
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Lina Perekhoda, Marharyta Suleiman, Illya Podolsky, Anton Semenets, Natalia Kobzar, Vitaliy Yaremenko, Olha Vislous, Victoriya Georgiyants, Sergiy Kovalenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






