Lavandula Angustifolia Mill. of Ukrainian origin: a comparative study of the chemical composition and antimicrobial potential of herb extracts
DOI:
https://doi.org/10.15587/2519-4852.2024.313236Keywords:
Lavandula angustifolia, extracts, chemical composition, antimicrobial activity, antifungal activity, biofilm formation of microbial culturesAbstract
Provide updated data on the antimicrobial activity and chemical composition of original dry extracts from Lavandula angustifolia herb of Ukrainian origin.
The aim – an experimental comparative study of the chemical profile and antimicrobial activity of the original dry extracts of Lavandula angustifolia herb and their effect on the ability to destroy biofilms of microbial cultures or prevent their formation in vitro.
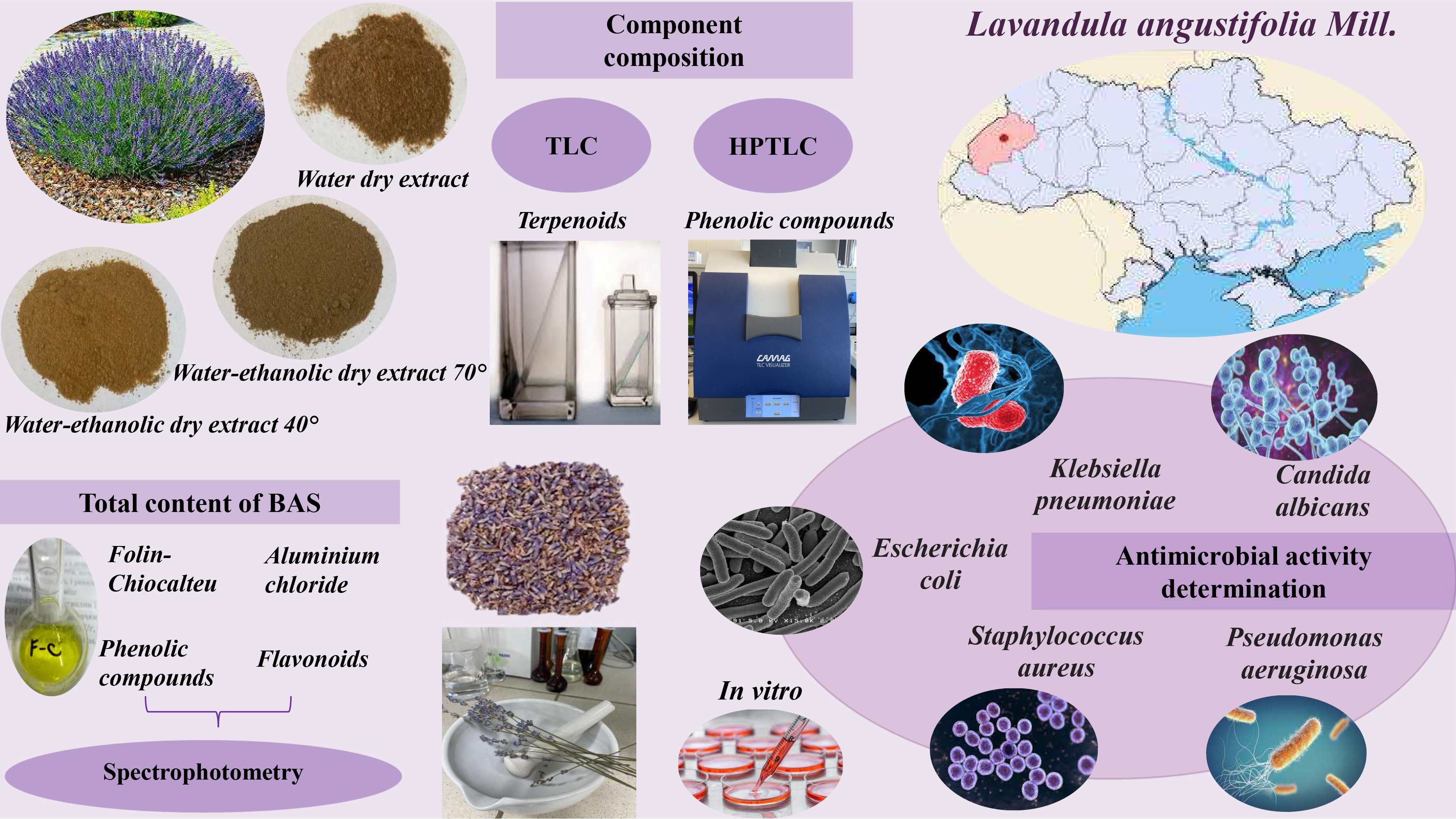
Materials and methods. The objects of the study are dry extracts obtained from the lavender herb with purified water and ethanol solutions (40 and 70 %). The main biologically active substances (BAS) of the extracts were determined by the Thin-layer chromatography and Absorption spectrophotometry methods. The microbiological properties of the test samples of the investigated plant extracts were studied in vitro by the two-fold serial dilutions method. The ability of microorganisms to form a biofilm was determined by the method of adhesion to polystyrene in flat-bottomed plastic plates. The optical density of the initial bacterial suspension was measured on the Densi-La-Meter device, and the density of inoculated bacterial cells on the Multiskan EX photometer at a wavelength of 540 nm. The study of the antimicrobial activity of water and ethanol extracts of lavender herb in a wide range of concentrations was carried out by the agar diffusion method in the "wells" modification, which is commonly used in microbiological practice.
Results. Water and water-ethanol extracts of lavender of Ukrainian origin were obtained. Terpenoids (linalool, linylyl acetate and traces of 1,8-cineol), flavonoids (hyperoside, isoquercitrin), and hydroxycinnamic acids (rosmarinic, chlorogenic acids) were identified in the extracts. The total content of phenolic compounds is 2.02–2.60 mg/g, flavonoids – 1.46–3.17 mg/g. The largest amount of BAS was extracted with 70 % ethanol. According to the results of experimental studies, the extracts of the lavender herb, obtained by extraction with a water-ethanol solution (40 and 70 % ethanol) at a concentration of 1 mg/ml, have antimicrobial properties against a wide range of infectious agents (S. aureus, E. coli, K. pneumoniae, P. aeruginosa, C. albicans). Studies of the influence of test samples of lavender extracts at a concentration of 1 mg/ml on the ability of microorganisms (S. aureus, E. coli, K. pneumoniae, P. aeruginosa) to form biofilms demonstrated that the highest inhibitory activity against biofilm formation was found in the case of the action of test of a sample of phytoextract obtained by extraction with a water-ethanol solution (40 % ethanol), which accounted for S. aureus ‒ 57.8 %, P. aeruginosa – 66.7 %. A wide spectrum of antimicrobial action was established for the tested lavender phytoextracts under the conditions of application of the concentration range of 10-60 μg/ml. The best spectrum of antimicrobial action and the highest activity corresponds to the lavender extract, obtained by extraction with 70 % ethanol, with the effect depending on the concentration.
Conclusion. The lavender herb of Ukrainian origin is a promising and affordable source of potential antimicrobial active pharmaceutical ingredients (API). Water-ethanol lavender extract (70 % ethanol), according to research results, has shown high antimicrobial and antifungal potential. According to preliminary data, antimicrobial activity correlates with the content of phenolic compounds. The obtained results may be useful for the search for original substances for the complex correction of symptoms of neurological deficits of infectious etiology
Supporting Agency
- The Ministry of Health of Ukraine provided funds for this study as part of the funding of a scientific topic: “Research of original substances for the correction of neurological deficits symptoms, prediction and assessment of factors affecting the mechanisms of action”, 2023-2025; №: 0123U101751
References
- Kremenchuk, R. I. (2017). Fitonomiia ta suchasnyi stan taksonomii lavandy (Lavandula L.). Suchasnyi stan ta harmonizatsiia nazv kulturnykh roslyn u systemi UPOV. Vinnytsia. Nilan-LTD, 26–27.
- Adaszyńska-Skwirzyńska, M., Dzięcioł, M. (2017). Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Natural Product Research, 31 (21), 2575–2580. https://doi.org/10.1080/14786419.2017.1320792
- Batiha, G. E.-S., Teibo, J. O., Wasef, L., Shaheen, H. M., Akomolafe, A. P., Teibo, T. K. A., Al-kuraishy, H. M., Al-Garbeeb, A. I., Alexiou, A., Papadakis, M. (2023). A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg’s Archives of Pharmacology, 396 (5), 877–900. https://doi.org/10.1007/s00210-023-02392-x
- Kalam, M. A., Habib, A., Arzoo, K. S., Ahmad, W., Ahmad, R., Avid, M. (2024). Khuzāma (Lavandula angustifolia Mill.): Pharmacological Action and Therapeutic Uses in Perspective of Unani Medicine: A Review. Journal of Complementary and Alternative Medical Research, 25 (1), 18–25. https://doi.org/10.9734/jocamr/2024/v25i1511
- Prusinowska, R., Śmigielski, K. B. (2014). Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review. Herba Polonica, 60 (2), 56–66. https://doi.org/10.2478/hepo-2014-0010
- Caputo, L., Souza, L., Alloisio, S., Cornara, L., De Feo, V. (2016). Coriandrum sativum and Lavandula angustifolia Essential Oils: Chemical Composition and Activity on Central Nervous System. International Journal of Molecular Sciences, 17 (12), 1999. https://doi.org/10.3390/ijms17121999
- Zaringhalam, J., Shams, J., Rezazadeh, S., Manaheji, H., Akhondzadeh, S., Asefifar, F. (2010). Role of the methanolic extracts of Boswellia serrata and Lavandula angustifolia on apomorphine induced ejaculation in male Wistar rats. Journal of Medicinal Plants Research, 4, 1073–1080.
- Slighoua, M., Mahdi, I., Ez-Zahra Amrati, F., Boucetta, N., Cristo, F. D., Boukhira, S. et al. (2022). Pharmacological effects of Lavandula officinalis Chaix and its polyphenols: Focus on their in vivo estrogenic and anti-inflammatory properties. South African Journal of Botany, 146, 354–364. https://doi.org/10.1016/j.sajb.2021.11.014
- Li, M., Cao, X., Yan, H., Wang, M., Tashibolati, A., Maiwulanjiang, M. (2022). Integrating Zebrafish Model to Screen Active Ingredients and Network Pharmacology Methods to Explore the Mechanism of Lavandula angustifolia Therapy for Alzheimer’s Disease. ChemistrySelect, 7 (28). https://doi.org/10.1002/slct.202201364
- Barut Gök, S., Erdoğdu, Y. (2024). Chemical composition and antimicrobial activity of essential oils from six lavender (Lavandula angustifolia Mill.) cultivars. Plant, Soil and Environment, 70 (2), 111–123. https://doi.org/10.17221/438/2023-pse
- Jianu, C., Pop, G., Lukinich-Gruia, A., Horhat, F. (2013). Chemical Composition and Antimicrobial Activity of Essential Oils of Lavender (Lavandula angustifolia) and Lavandin (Lavandula x intermedia) Grown in Western Romania. International Journal of Agriculture and Biology, 15, 772–776.
- Torumkuney, D., Bratus, E., Yuvko, O., Pertseva, T., Morrissey, I. (2020). Results from the Survey of Antibiotic Resistance (SOAR) 2016–17 in Ukraine: data based on CLSI, EUCAST (dose-specific) and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints. Journal of Antimicrobial Chemotherapy, 75 (1), i100–i111. https://doi.org/10.1093/jac/dkaa087
- Müller, A., Schmidt, L. (2021). Assessing the Development of Fungicide Resistance in Microorganisms: Current Status and Future Perspectives. Frontiers in Microbiology, 12, 589.
- Villalba, N., Ma, Y., Gahan, S. A., Joly-Amado, A., Spence, S., Yang, X. et al. (2023). Lung infection by Pseudomonas aeruginosa induces neuroinflammation and blood–brain barrier dysfunction in mice. Journal of Neuroinflammation, 20 (1). https://doi.org/10.1186/s12974-023-02817-7
- McLoughlin, A., Rochfort, K. D., McDonnell, C. J., Kerrigan, S. W., Cummins, P. M. (2016). Staphylococcus aureus-mediated blood-brain barrier injury: anin vitrohuman brain microvascular endothelial cell model. Cellular Microbiology, 19 (3), e12664. https://doi.org/10.1111/cmi.12664
- Kielian, T., Hickey, W. F. (2000). Proinflammatory Cytokine, Chemokine, and Cellular Adhesion Molecule Expression during the Acute Phase of Experimental Brain Abscess Development. The American Journal of Pathology, 157 (2), 647–658. https://doi.org/10.1016/s0002-9440(10)64575-0
- Weissenborn, K., Donnerstag, F., Kielstein, J. T., Heeren, M., Worthmann, H., Hecker, H. et al. (2012). Neurologic manifestations of E coli infection-induced hemolytic-uremic syndrome in adults. Neurology, 79 (14), 1466–1473. https://doi.org/10.1212/wnl.0b013e31826d5f26
- Fang, C. T., Chen, Y. C., Chang, S. C., Sau, W. Y., Luh, K. T. (2000). Klebsiella pneumoniae meningitis: timing of antimicrobial therapy and prognosis. QJM, 93, 45–53.
- Kwiatkowski, P., Łopusiewicz, Ł., Kostek, M., Drozłowska, E., Pruss, A., Wojciuk, B. et al. (2019). The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules, 25 (1), 95. https://doi.org/10.3390/molecules25010095
- Gismondi, A., Di Marco, G., Redi, E. L., Ferrucci, L., Cantonetti, M., Canini, A. (2021). The antimicrobial activity of Lavandula angustifolia Mill. essential oil against Staphylococcus species in a hospital environment. Journal of Herbal Medicine, 26, 100426. https://doi.org/10.1016/j.hermed.2021.100426
- Ramić, D., Bucar, F., Kunej, U., Dogša, I., Klančnik, A., Smole Možina, S. (2021). Antibiofilm Potential of Lavandula Preparations against Campylobacter jejuni. Applied and Environmental Microbiology, 87 (19). https://doi.org/10.1128/aem.01099-21
- El-Tarabily, K. A., El-Saadony, M. T., Alagawany, M., Arif, M., Batiha, G. E., Khafaga, A. F. et al. (2021). Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi Journal of Biological Sciences, 28 (9), 5145–5156. https://doi.org/10.1016/j.sjbs.2021.05.033
- Slimani, C., Sqalli, H., Rais, C., Farah, A., Lazraq, A., GHADRAOUI, L. E., Belmalha, S., Echchgadda, G. (2022). Chemical composition and evaluation of biological effects of essential oil and aqueous extract of Lavandula angustifolia L. Notulae Scientia Biologicae, 14 (1), 11172. https://doi.org/10.15835/nsb14111172
- Moon, T., Wilkinson, J., Cavanagh, H. (2006). Antibacterial activity of essential oils, hydrosols and plant extracts from Australian grown Lavandula spp. International Journal of Aromatherapy, 16 (1), 9–14. https://doi.org/10.1016/j.ijat.2006.01.007
- Zenão, S., Aires, A., Dias, C., Saavedra, M. J., Fernandes, C. (2017). Antibacterial potential of Urtica dioica and Lavandula angustifolia extracts against methicillin resistant Staphylococcus aureus isolated from diabetic foot ulcers. Journal of Herbal Medicine, 10, 53–58. https://doi.org/10.1016/j.hermed.2017.05.003
- Abdelhamid, A. G., Yousef, A. E. (2023). Combating Bacterial Biofilms: Current and Emerging Antibiofilm Strategies for Treating Persistent Infections. Antibiotics, 12 (6), 1005. https://doi.org/10.3390/antibiotics12061005
- Choi, V., Rohn, J. L., Stoodley, P., Carugo, D., Stride, E. (2023). Drug delivery strategies for antibiofilm therapy. Nature Reviews Microbiology, 21 (9), 555–572. https://doi.org/10.1038/s41579-023-00905-2
- Mączka, W., Duda-Madej, A., Grabarczyk, M., Wińska, K. (2022). Natural Compounds in the Battle against Microorganisms – Linalool. Molecules, 27 (20), 6928. https://doi.org/10.3390/molecules27206928
- Gupta, A., Singh, P. P., Singh, P., Singh, K., Singh, A. V., Singh, S. K., Kumar, A. (2019). Medicinal Plants Under Climate Change: Impacts on Pharmaceutical Properties of Plants. Climate Change and Agricultural Ecosystems. Woodhead Publishing, 181–209. https://doi.org/10.1016/b978-0-12-816483-9.00008-6
- Harna, S. V. (2015). The rational use of the natural extracts. Zbirnyk naukovykh prats spivrobitnykiv NMAPO im. P. L. Shupyka, 24 (5), 306–311. Available at: http://nbuv.gov.ua/UJRN/Znpsnmapo_2015_24(5)__60
- Mykhailenko, O., Hurina, V., Ivanauskas, L., Marksa, M., Skybitska, M., Kovalenko, O. et al. (2024). Lavandula angustifolia Herb from Ukraine: Comparative Chemical Profile and in vitro Antioxidant Activity. Chemistry & Biodiversity, 21 (9). https://doi.org/10.1002/cbdv.202400640
- European Pharmacopoeia (Ph. Eur.) Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition Last accessed: 23.04.2024
- Ivanauskas, L., Uminska, K., Gudžinskas, Z., Heinrich, M., Georgiyants, V., Kozurak, A., Mykhailenko, O. (2023). Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine. Plants, 13 (1), 120. https://doi.org/10.3390/plants13010120
- Derzhavna Farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- Mykhailenko, O., Bezruk, I., Volochai, V., Mishchenko, V., Ivanauskas, L., Georgiyants, V. (2022). Phytochemical Analysis and Antioxidant Activity of Crocus speciosus Leaves. Phyton, 91 (1), 207–221. https://doi.org/10.32604/phyton.2022.016458
- Volianskyi, Yu. L., Hrytsenko, I. S., Shyrobokov, V. P. (2004). Vyvchennia spetsyfichnoi aktyvnosti protymikrobnykh likarskykh zasobiv. Kyiv: DFTs MOZ Ukrainy, 38.
- Vyznachennia chutlyvosti mikroorhanizmiv do antybakterialnykh preparativ (2007). Metodychni vkazivky.
- Hryhoriv, H., Mariutsa, I., Kovalenko, S. M., Sidorenko, L., Perekhoda, L., Filimonova, N. et al. (2021). Structural modification of ciprofloxacin and norfloxacin for searching new antibiotics to combat drug-resistant bacteria. ScienceRise: Pharmaceutical Science, 5 (33), 4–11. https://doi.org/10.15587/2519-4852.2021.242997
- Oliveira, N. M., Martinez-Garcia, E., Xavier, J., Durham, W. M., Kolter, R., Kim, W., Foster, K. R. (2015). Correction: Biofilm Formation As a Response to Ecological Competition. PLOS Biology, 13 (8), e1002232. https://doi.org/10.1371/journal.pbio.1002232
- Kukhtyn, M., Berhilevych, O., Kravcheniuk, K., Shynkaruk, O., Horyuk, Y., Semaniuk, N. (2017). Formation of biofilms on dairy equipment and the influence of disinfectants on them. Eastern-European Journal of Enterprise Technologies, 5 (11 (89)), 26–33. https://doi.org/10.15587/1729-4061.2017.110488
- Coyle, M. B. (2005). Manual of Antimicrobial Susceptibility Testing. American Society for Microbiology. Washington: American Society for Microbiology, 236.
- McFarland, J. (1907). The nephelometer:an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA: The Journal of the American Medical Association, XLIX (14), 1176–1178. https://doi.org/10.1001/jama.1907.25320140022001f
- Kutasevych, Y., Dzhoraieva, S. K., Kondakova, G. K., Khoroshun, E. M., Lyapunov, N. A., Goncharenko, V. V. et al. (2023). Determination of biofilm formation ability of representatives of microbiocenose of battle wounds. Dermatology and Venerology, 3, 13–17. https://doi.org/10.33743/2308-1066-2023-3-13-17
- Malcheva, B. Z., Naskova, P., Grigorova-Pesheva, B., Plamenov, D., Yordanova, M., Kostadinova-Slaveva, A. et al. (2024). Investigation of Lavandula angustifolia Mill. extracts as anti-Escherichia coli agents and microbial additives: are they an alternative to enrichment, decontaminating and deodorizing agents for organic soil improvers? Soil Science Annual, 75 (1), 1–9. https://doi.org/10.37501/soilsa/186455
- Kryvtsov, M. V., Király, J., Koščová, J., Kostenko, Ye. Ya., Bubnov, R. V., Spivak, M. Ya. (2020). Determination of biofilm formation and associated gene detection in Staphylococcus genus isolated from the oral cavity under inflammatory periodontal diseases. Studia Biologica, 14 (3), 49–64. https://doi.org/10.30970/sbi.1403.627
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Olena Bogatyrova, Viktoriia Hurina, Olga Naboka, Nataliia Filimonova, Svitlana Dzhoraieva, Olha Mykhailenko, Victoriya Georgiyants

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






