Analyst qualification for compliance with normal analytical practice for pipette use
DOI:
https://doi.org/10.15587/2519-4852.2024.318519Keywords:
Mohr pipette, proficiency testing, measurement uncertainty, precision, State Pharmacopeia of UkraineAbstract
Aim. This work aimed to assess the proficiency of routine medicine quality control analysts for compliance with the normal analytical practice (NAP) requirements for the standard pipette aliquoting analytical procedure.
Materials and methods. Certified 2 mL Mohr pipettes provided by the proficiency testing provider; a gravimetric procedure for determining the delivered volume and the corresponding equipment that meets the ISO 4787:2021 requirements; methods of mathematical statistics.
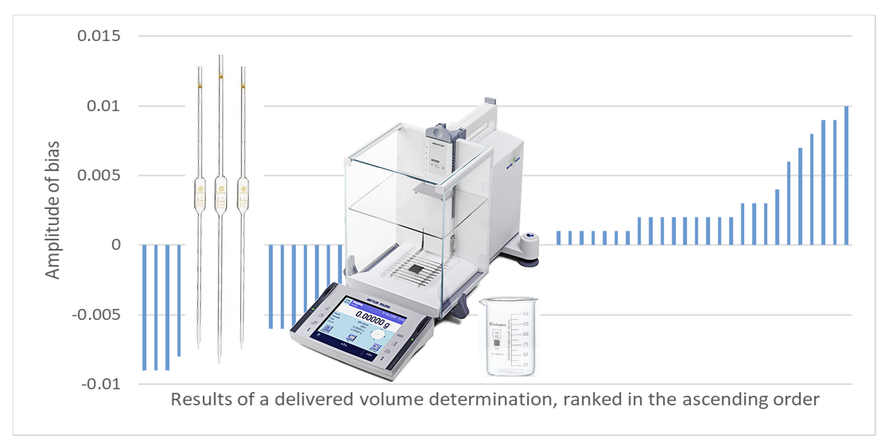
Results and discussion. The participants conducted five measurements of the delivered volume using the gravi-metric method. The acceptance criteria for the results were developed to ensure reliable verification of volumetric glassware, where the random analyst’s error had to be insignificant compared to the requirements for the error of the volumetric glassware, as well as compliance with NAP in routine analysis, particularly regarding individual deviations. A total of 64 analysts from 22 laboratories participated in the testing. Of these, 61 analysts achieved satisfactory results by all criteria. For 44 % of the participants, the deviations from the nominal value were signif-icant as per the ISO requirements for Class A pipettes. Individual and average deviations from the nominal value were calculated and analyzed, allowing for assessing the participant results’ precision and the correctness of their decisions regarding the ISO requirements for measuring equipment. The accuracy of the participants’ calculations, the instruments used, and the testing conditions were evaluated. Issues with calculations, rounding of results, and non-compliance with the requirements concerning metrological balance qualification, thermometer calibration, laboratory environmental conditions, and pipette condition evaluation were revealed.
Conclusions. Findings indicate that participants can meet NAP requirements and perform pipette verification with high reliability, which aligns with the key analyst qualification requirements. The results confirm that for personnel qualification, it is necessary to use pipettes with certified delivered volume with acceptable uncertainty. The devel-oped testing procedure can be used for intra-laboratory proficiency testing
References
- General European OMCL Network (GEON). PA/PH/OMCL (15) 50 R8 (2020). Alternatives to Proficiency Testing Schemes (PTS). Available at: https://www.edqm.eu/documents/52006/0/Web_publication_EDQM_Alternatives+to+PTS+OMCL+15+50+R8.pdf/0ecf9848-920c-cc74-ef59-2ad6ca606686?t=1670595816655
- FDA Office of Regulatory Affairs ORA Laboratory manual volume II (2019). Ensuring the quality of test results. Document number: ORA-LAB. 5.9. Available at: https://www.fda.gov/media/73979/download?attachment
- General European OMCL Network (GEON). PA/PH/OMCL (13) 113 R7 (2023). Evaluation and reporting of results – Core document. Available at: https://www.edqm.eu/en/d/129021?p_l_back_url=%2Fen%2Fquality-management-qm-documents%3Fq%3Dcompliance
- Williams, A., Magnusson, B. (Eds.) (2021). Eurachem/CITAC Guide: Use of uncertainty information in compliance assessment. Available at: https://www.eurachem.org/images/stories/Guides/pdf/MUC2021_P1_EN.pdf
- European Pharmacopoeia 11th edition (2022). 1. General Notices. 1.5.1.9. Tests and Assays. Strasbourg: European Directorate for the Quality of Medicines. Available at: https://pheur.edqm.eu/home
- USP General Notices and Requirements. 4.10.20. Acceptance Criteria, 7.10. Interpretation of Requirements (2023). The United States Pharmacopoeia 43rd edition. Available at: https://online.uspnf.com/uspnf
- The international pharmacopoeia. General requirements (2022). Geneva: World Health Organization. Available at: https://digicollections.net/phint/2022/index.html#p/home
- Annex 4: WHO good practices for pharmaceutical quality control laboratories, 6.7 Measurement uncertainty. WHO Technical Report Series, No. 1052. WHO Expert Committee on Specifications for Pharmaceutical Preparations: fifty-seventh report (2024). Geneva: World Health Organization, 193–195. Available at: https://iris.who.int/bitstream/handle/10665/376607/9789240091030-eng.pdf?sequence=1
- Technical Guide for the Elaboration of Monographs (2022). European Directorate for the Quality of Medicines & HealthCare, Council of Europe, Strasbourg. Available at: https://www.edqm.eu/en/-/new-edition-of-the-technical-guide-for-the-elaboration-of-ph.-eur.-monographs-ready-for-publication
- 3.N.2. Validation of analytical procedures (2024). The State Pharmacopoeia of Ukraine. Supplement 7.2. Kharkiv: State Enterprise "Ukrainian Scientific Pharmacopeial Centre for Quality of Medicines", 126–241.
- De Oliveira Pereira, C. E., Souza, M. A. C. e, Pianetti, G. A., de Souza, S. V. C. (2017). Overview of proficiency testing provision in pharmaceutical area in Brazil and an educational scheme for determining mefenamic acid in raw materials. Accreditation and Quality Assurance, 22 (2), 63–72. https://doi.org/10.1007/s00769-017-1251-2
- Technical Review of MHRA Analytical Quality by Design Project (2019). Medicines and Healthcare products Regulatory Agency. Available at: https://assets.publishing.service.gov.uk/media/5cfa8205ed915d736df4cb98/AQbD_Technical_Document_-_Final_04_June_2019.pdf
- Ellison, S., Williams, A. (Eds.) (2012). Eurachem/CITAC Guide: Quantifying uncertainty in analytical measurement. Available at: https://www.eurachem.org/images/stories/Guides/pdf/QUAM2012_P1.pdf
- Leontiev, D., Gryzodub, O., Arkhipova, N., Zvolinskaya, N., Dotsenko, T., Denisenko, N. (2003). Reproducibility of pharmacopoeial HPLC assay methods in inter-laboratory trials: role of sample preparation uncertainty. Farmakom, 4, 4–12. Available at: https://sphu.org/en/journal-pharmacom/archive-2003
- Gryzodub, O., Zvolinskaya, N., Arkhipova, N., Leontiev, D., Denysenko, N., Dotsenko, T. (2004). Reproducibility of pharmacopoeial spectrophotometrical procedures of medication assays in different laboratories. Farmakom, 2, 20–34. Available at: https://sphu.org/en/journal-pharmacom/archive-2004
- Asmolov, V., Leontiev, D., Volovyk, N., Gryzodub, O. (2023). Personnel Testing for Compliance with Normal Analytical Practice: Aliquot Taking by Pipette. Actual problems of quality, management, and economy in Pharmacy and healthcare. Kharkiv, 94–96. Available at: https://www.researchgate.net/publication/375891181_Personnel_Testing_for_Compliance_with_Normal_Analytical_Practic_Aliquot_Taking_by_Pipette
- General European OMCL Network (GEON). Quality management document PA/PH/OMCL (20) 95 R2 (2021). Qualification and re-qualification of personnel involved in laboratory activities. Available at: https://www.edqm.eu/documents/52006/128968/qualification-and-re-qualification-of-personnel-involved-in-laboratory-activities.pdf/c922e1a5-863d-7e78-a784-d2a77f3141f5?t=1628491791670
- Asmolov, V., Leontiev, D., Chykalova, S., Volovyk, N., Gryzodub, O. (2023). Mohr pipette calibration as a test item for professional testing of laboratories. Modern chemistry of medicines: materials of international modern conference. Kharkiv, 9‒11. Available at: https://www.researchgate.net/publication/376830295_MORH_PIPETTE_CALIBRATION_AS_A_TEST_ITEM_FOR_PROFESSIONAL_TESTING_OF_LABORATORIES
- 3.N.3. Quality assurance (2024). The State Pharmacopoeia of Ukraine: 2nd edition, Supplement 7.2. Kharkiv: State Enterprise "Ukrainian Scientific Pharmacopeial Centre for Quality of Medicines", 242–261.
- 3.N.1. Statistical analysis of chemical experiment results (2024). The State Pharmacopoeia of Ukraine: 2nd edition, Supplement 7.2. Kharkiv: State Enterprise "Ukrainian Scientific Pharmacopeial Centre for Quality of Medicines", 35–125.
- ISO 4787:2021. Laboratory glass and plastic ware – Volumetric instruments – Methods for testing of capacity and for use (2021). Available at: https://www.iso.org/ru/standard/74926.html
- ISO 648:2008. Laboratory glassware. Single-volume pipettes (2022). Available at: https://www.iso.org/ru/standard/44142.html
- Komarova, Y., Leontiev, D., Gryzodub, O. (2014). Quality analysis results when performing basic operations of sample preparation, volumetric pipettes with one mark. Farmakom, 4, 13–22. Available at: https://sphu.org/en/journal-pharmacom/archive-2014
- Sur, S., Gryzodub, O., Gubar, S., Leontiev, D., Zvolinska, N., Denisenko, N., Murashko, A. (2009). Data of an assay of the test sample of the substance of sodium acetate trihydrate by drug quality control laboratories in the 6th round of programs of professional testing. Farmakom, 4, 11–20. Available at: https://sphu.org/en/journal-pharmacom/archive-2009
- General European OMCL Network (GEON). Quality management document PA/PH/OMCL (12)77 R12 (2023). Qualification of equipment. Qualification of balances. Available at: https://www.edqm.eu/documents/52006/128968/omcl-qualification-of-balances.pdf/79404120-a71a-58d6-d597-305cd69e112a?t=1628491787423
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Dmytro Leontiev, Svitlana Chykalova, Vitalii Asmolov, Natalia Volovyk, Vasyl Petrus, Oleksandr Gryzodub

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






