Influence of a new combined production based on dense carrot root extract and quercetin on the morphological and proliferative properties of l929 line fibroblasts in cell culture
DOI:
https://doi.org/10.15587/2519-4852.2024.319401Keywords:
L929 cell line, cytotoxic effect, dense carrot root extract, quercetinAbstract
At the first stage of studying the pharmacological properties of substances, the general cytotoxic effect is determined based on the assessment of viability, morphological integrity and functional activity of cells. One of the standard cell lines often used in cytotoxicity tests is the L929 line.
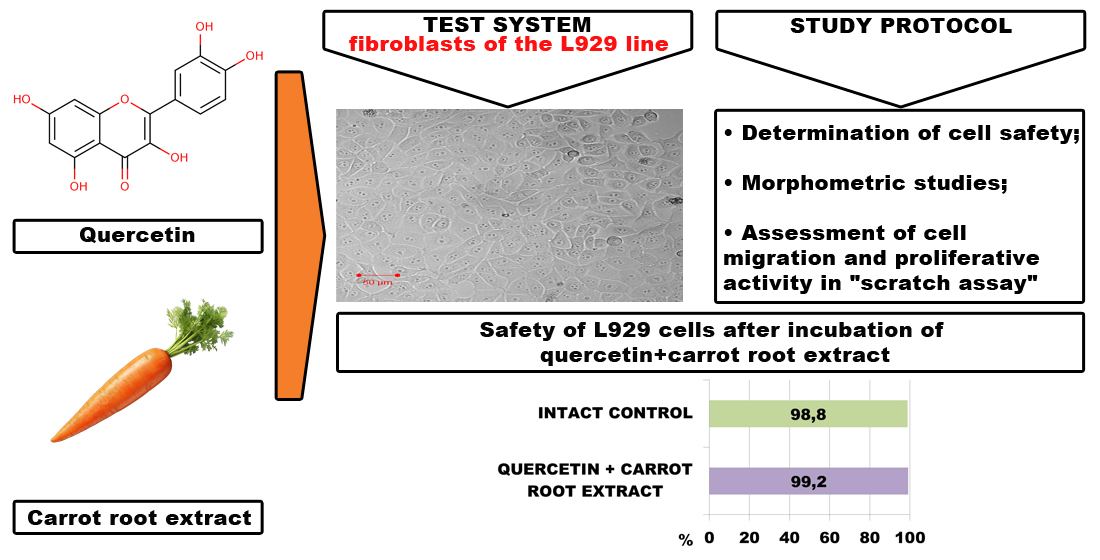
Studying the ability of a combined lipid-lowering agent based on dense carrot root extract and quercetin to influence the morphological and functional properties of fibroblasts of the L929 line in cell culture was the aim of this study.
Materials and methods: Determination of the cytotoxicity of the drug under study was carried out in a cell culture of the L929 line at a concentration of 40, 100, and 200 mg/ml. The safety of cells in the resulting suspension was assessed using staining with a 0,4% trypan blue solution. Cell morphology and nuclear-cytoplasmic ratio were assessed in fixed culture preparations stained with hematoxylin-eosin. To assess the migration and proliferative activity of a monolayer culture, the “scratch” test was used.
Results: When L929 cells were incubated in a nutrient medium with the addition of the product, their viability and morphological properties were preserved; images of a monolayer of the culture of these cells under phase-contrast microscopy coincided with the quantitative analysis data. The absence of changes in the nuclear-cytoplasmic ratio was further evidence of the absence of toxic effects of the studied drug in relation to fibroblast culture. According to the experiment results, the migration and proliferative activity of L929 cells under the influence of the drug did not differ significantly from intact cells.
Conclusions: The results of the toxicological study showed that the combined product based on dense carrot root extract and quercetin does not have a cytotoxic effect on the L929 cell line culture and can be recommended for further preclinical research.
References
- Horváth, S. (1980). Cytotoxicity of drugs and diverse chemical agents to cell cultures. Toxicology, 16 (1), 59–66. https://doi.org/10.1016/0300-483x(80)90110-9
- Whelan, M., Eskes, C. (2016). Evolving the Principles and Practice of Validation for New Alternative Approaches to Toxicity Testing. Validation of Alternative Methods for Toxicity Testing, 387–399. https://doi.org/10.1007/978-3-319-33826-2_15
- Griesinger, C., Desprez, B., Coecke, S., Casey, W., Zuang, V. (2016). Validation of Alternative In Vitro Methods to Animal Testing: Concepts, Challenges, Processes and Tools. Validation of Alternative Methods for Toxicity Testing, 65–132. https://doi.org/10.1007/978-3-319-33826-2_4
- Bácskay, I., Nemes, D., Fenyvesi, F., Váradi, J., Vasvári, G., Fehér, P. et al. (2018). Role of Cytotoxicity Experiments in Pharmaceutical Development. Cytotoxicity. https://doi.org/10.5772/intechopen.72539
- Sazdova, I., Keremidarska-Markova, M., Chichova, M., Uzunov, B., Nikolaev, G., Mladenov, M. et al. (2022). Review of Cyanotoxicity Studies Based on Cell Cultures. Journal of Toxicology, 2022, 1–17. https://doi.org/10.1155/2022/5647178
- Segall, M. D., Barber, C. (2014). Addressing toxicity risk when designing and selecting compounds in early drug discovery. Drug Discovery Today, 19(5), 688–693. https://doi.org/10.1016/j.drudis.2014.01.006
- Róka, E., Ujhelyi, Z., Deli, M., Bocsik, A., Fenyvesi, É., Szente, L. et al. (2015). Evaluation of the Cytotoxicity of α-Cyclodextrin Derivatives on the Caco-2 Cell Line and Human Erythrocytes. Molecules, 20 (11), 20269–20285. https://doi.org/10.3390/molecules201119694
- Russell, W. M. S., Burch, R. L. (1959). The principles of humane experimental technique. London: Methuen & Co. Ltd., 238.
- Amelian, A., Wasilewska, K., Megias, D., Winnicka, K. (2017). Application of standard cell cultures and 3D in vitro tissue models as an effective tool in drug design and development. Pharmacological Reports, 69 (5), 861–870. https://doi.org/10.1016/j.pharep.2017.03.014
- Śliwka, L., Wiktorska, K., Suchocki, P., Milczarek, M., Mielczarek, S., Lubelska, K. et al. (2016). The Comparison of MTT and CVS Assays for the Assessment of Anticancer Agent Interactions. PLOS ONE, 11 (5), e0155772. https://doi.org/10.1371/journal.pone.0155772
- Kim, S. I., Kim, H. J., Lee, H.-J., Lee, K., Hong, D., Lim, H. et al. (2016). Application of a non-hazardous vital dye for cell counting with automated cell counters. Analytical Biochemistry, 492, 8–12. https://doi.org/10.1016/j.ab.2015.09.010
- Präbst, K., Engelhardt, H., Ringgeler, S.; Gilbert, D., Friedrich, O. (Eds.) (2017). Basic colorimetric proliferation assay: MTT, WST and Resazurin. In: editors. Cell Viability Assays. Methods in Molecular Biology. Vol. 1601. New York: Humana Press. https://doi.org/10.1007/978-1-4939-6960-9_1
- Ülker, M., Çelik, A. C. T., Yavuz, E., Kahvecioğlu, F., Ülker, H. E. (2021). Real-Time Analysis of Antiproliferative Effects of Mouthwashes Containing Alcohol, Sodium Fluoride, Cetylpyridinium Chloride, and Chlorhexidine In Vitro. BioMed Research International, 2021, 1–8. https://doi.org/10.1155/2021/2610122
- Dawid, C., Dunemann, F., Schwab, W., Nothnagel, T., Hofmann, T. (2015). Bioactive C17-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. Journal of Agricultural and Food Chemistry, 63 (42), 9211–9222. https://doi.org/10.1021/acs.jafc.5b04357
- Purup, S., Larsen, E., Christensen, L. P. (2009). Differential Effects of Falcarinol and Related Aliphatic C17-Polyacetylenes on Intestinal Cell Proliferation. Journal of Agricultural and Food Chemistry, 57 (18), 8290–8296. https://doi.org/10.1021/jf901503a
- Mandrich, L., Esposito, A. V., Costa, S., Caputo, E. (2023). Chemical Composition, Functional and Anticancer Properties of Carrot. Molecules, 28 (20), 7161. https://doi.org/10.3390/molecules28207161
- Superchi, S., Pini, D., Salvadori, P., Marinelli, F., Rainaldi, G., Zanelli, U., Nuti-Ronchi, V. (1993). Synthesis and toxicity to mammalian cells of the carrot dihydroisocoumarins. Chemical Research in Toxicology, 6 (1), 46–49. https://doi.org/10.1021/tx00031a007
- Sharma, M., Sharma, R., Jain, D. K. (2016). Nanotechnology Based Approaches for Enhancing Oral Bioavailability of Poorly Water Soluble Antihypertensive Drugs. Scientifica, 2016, 1–11. https://doi.org/10.1155/2016/8525679
- Yang, D., Wang, T., Long, M., Li, P. (2020). Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Medicine and Cellular Longevity, 2020, 1–13. https://doi.org/10.1155/2020/8825387
- Someya, Y., Saito, S., Takeda, S., Adachi, N., Kurosawa, A. (2024). Quercetin exhibits cytotoxicity in cancer cells by inducing two-ended DNA double-strand breaks. Biochemical and Biophysical Research Communications, 739, 150977. https://doi.org/10.1016/j.bbrc.2024.150977
- Kononenko, T., Chikitkina, V. (2024). Experimental determination of the conditionally therapeutic dose of a new combined hypolipidemic agent based on carrot root extract and quercetin on the model of acute hyperlipidemia. Fitoterapia, 2, 137–146. https://doi.org/10.32782/2522-9680-2024-2-137
- Idowu, B., Di Silvio, L. (2013). Principles of good laboratory practice (GLP) for in vitro cell culture applications. Standardisation in Cell and Tissue Engineering. Methods and Protocols, 127–147.
- Paziuk, D.-M. V., Zhuravel, I. O., Kyslychenko, O. A., Horiacha, L. M. (2024). Pat. No. 120675 UA. Zasib z antybakterialnoiu ta protyhrybkovoiu aktyvnistiu z morkvy posivnoi. No. u 2017 05682; declareted: 09.06.2017; published: 10.11.2017, Bul. No. 21.
- Kyslychenko, O. A. (2020). Pharmacognostic study of plants for the development of drugs for the treatment of cardivascular diseases. [Doctoral dissertation; National University of Pharmacy].
- Strober, W. (2015). Trypan Blue Exclusion Test of Cell Viability. Current Protocols in Immunology, 111 (1). https://doi.org/10.1002/0471142735.ima03bs111
- Turgeon, M. L. (2018). Clinical hematology: Theory and procedure. Philadelphia: Wolters Kluwer, 800.
- Denker, S. P., Barber, D. L. (2002). Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. The Journal of Cell Biology, 159 (6), 1087–1096. https://doi.org/10.1083/jcb.200208050
- Andres, S., Pevny, S., Ziegenhagen, R., Bakhiya, N., Schäfer, B., Hirsch‐Ernst, K. I., Lampen, A. (2017). Safety Aspects of the Use of Quercetin as a Dietary Supplement. Molecular Nutrition & Food Research, 62 (1). https://doi.org/10.1002/mnfr.201700447
- Harwood, M., Danielewska-Nikiel, B., Borzelleca, J. F., Flamm, G. W., Williams, G. M., Lines, T. C. (2007). A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food and Chemical Toxicology, 45 (11), 2179–2205. https://doi.org/10.1016/j.fct.2007.05.015
- Cialdella-Kam, L., Nieman, D. C., Sha, W., Meaney, M. P., Knab, A. M., Shanely, R. A. (2012). Dose–response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults. British Journal of Nutrition, 109 (11), 1923–1933. https://doi.org/10.1017/s0007114512003972
- Sodimbaku, V., Pujari, L., Mullangi, R., Marri, S. (2016). Carrot (Daucus carota L.): Nephroprotective against gentamicin-induced nephrotoxicity in rats. Indian Journal of Pharmacology, 48 (2), 122–127. https://doi.org/10.4103/0253-7613.178822
- Edgar, B. A., Orr-Weaver, T. L. (2001). Endoreplication Cell Cycles. Cell, 105 (3), 297–306. https://doi.org/10.1016/s0092-8674(01)00334-8
- Balachandra, S., Sarkar, S., Amodeo, A. A. (2022). The Nuclear-to-Cytoplasmic Ratio: Coupling DNA Content to Cell Size, Cell Cycle, and Biosynthetic Capacity. Annual Review of Genetics, 56 (1), 165–185. https://doi.org/10.1146/annurev-genet-080320-030537
- Tollis, S., Rizzotto, A., Pham, N. T., Koivukoski, S., Sivakumar, A., Shave, S. et al. (2022). Chemical Interrogation of Nuclear Size Identifies Compounds with Cancer Cell Line-Specific Effects on Migration and Invasion. ACS Chemical Biology, 17 (3), 680–700. https://doi.org/10.1021/acschembio.2c00004
- Ammann, K. R., DeCook, K. J., Li, M., Slepian, M. J. (2019). Migration versus proliferation as contributor to in vitro wound healing of vascular endothelial and smooth muscle cells. Experimental Cell Research, 376 (1), 58–66. https://doi.org/10.1016/j.yexcr.2019.01.011
- Cory, G. (2011). Scratch-Wound Assay. Cell Migration, 25–30. https://doi.org/10.1007/978-1-61779-207-6_2
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Tetiana Kononenko, Viktoria Ustichenko, Galyna Bozhok, Valentyna Chikitkina, Rymma Yeromenko, Inna Kovalevska, Viktoriia Verkhovod

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






