Synthesis of new 4,4'-(1H-1,2,3-triazole)-bis(1H-pyrazol-5-ols) and prospects for their study as potential antitumor agents
DOI:
https://doi.org/10.15587/2519-4852.2025.327116Keywords:
1,2,3-triazole, pyrazole, synthesis, anticancer activity, docking studiesAbstract
The aim of our work is to develop an efficient synthesis of a series of novel 4,4'-(1H-1,2,3-triazol)bis(1H-pyrazol-5-ols), synthesize the target substances, and perform molecular docking focusing on the interaction of the synthesized compounds with the active sites of known cytostatics targeting various stages of oncogenesis.
Materials and methods. The structure and purity of the obtained substances were confirmed by 1H NMR spectroscopy, 13C NMR spectroscopy and LC/MS. Docking studies were performed for the substances synthesized using Autodock 4.2 software.
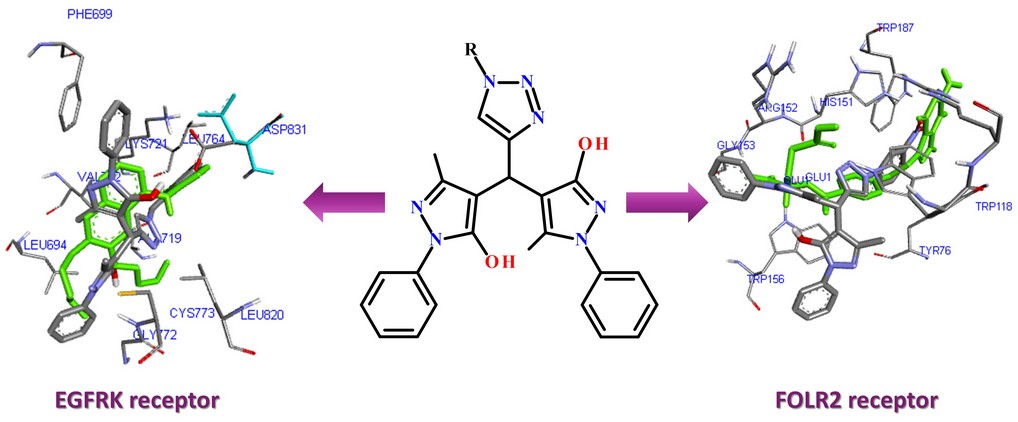
Results and discussion. A series of novel 4,4'-(1H-1,2,3-triazol)bis(1H-pyrazol-5-ols) were synthesized via a tandem Knoevenagel–Michael reaction from two equivalents of 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one with various 1,2,3-triazole aldehydes catalyzed by ammonium acetate in ethanol in high yields. As a result of the analysis of the array of docking computations and a detailed analysis of the geometric arrangement in the active sites of tumour targets (C-abl kinase, deoxycytidine kinase (dCK), CSF1 receptor, EGFRK receptor, FOLR2 receptor, it was found that the synthesized derivatives may have antitumor effects through the mechanism of inhibition of the EGFRK receptor.
Conclusions. According to the molecular docking data, the newly synthesized derivatives 4,4'-((1H-1,2,3-triazol-4-yl)methylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) may have an antitumor effect through the mechanism of EGFRK receptor inhibition
Supporting Agency
- This project has received funding through the MSCA4 Ukraine project, which is funded by the European Union.
References
- Schönthal, A. H. (2007). Direct non-cyclooxygenase-2 targets of celecoxib and their potential relevance for cancer therapy. British Journal of Cancer, 97 (11), 1465–1468. https://doi.org/10.1038/sj.bjc.6604049
- Pawar, V., Shastri, L. A., Gudimani, P., Joshi, S., Sunagar, V. (2022). Synthesis, characterization and molecular docking of novel lonazolac analogues 3-(3-hydroxy-5-methyl-1H-pyrazol-4-yl)-3-arylpropanoic acid derivatives: Highly potential COX-1/COX-2, matrix metalloproteinase and protein denaturation inhibitors. Journal of Molecular Structure, 1260, 132782. https://doi.org/10.1016/j.molstruc.2022.132782
- Jia, T., Cai, M., Wang, Z., Chen, T. (2023). Anticancer effect of crizotinib on osteosarcoma cells by targeting c-Met signaling pathway. Cellular and Molecular Biology, 69 (5), 174–178. https://doi.org/10.14715/cmb/2023.69.5.27
- Abdul Rahman, S. M., Bhatti, J. S., Thareja, S., Monga, V. (2023). Current development of 1,2,3-triazole derived potential antimalarial scaffolds: Structure- activity relationship (SAR) and bioactive compounds. European Journal of Medicinal Chemistry, 259, 115699. https://doi.org/10.1016/j.ejmech.2023.115699
- Dunn, G. L., Hoover, J. R. E., Berges, D. A., Taggart, J. J., Davis, L. D., Dietz, E. M. et al. (1976). Orally active 7-phenylglycyl cephalosporins. Structure-activity studies related to cefatrizine (SK&F 60771). The Journal of Antibiotics, 29 (1), 65–80. https://doi.org/10.7164/antibiotics.29.65
- Gallagher, J. C., Satlin, M. J., Elabor, A., Saraiya, N., McCreary, E. K., Molnar, E. et al. (2018). Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: A Multicenter Study. Open Forum Infectious Diseases, 5 (11). https://doi.org/10.1093/ofid/ofy280
- Xu, Z., Zhao, S.-J., Liu, Y. (2019). 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. European Journal of Medicinal Chemistry, 183, 111700. https://doi.org/10.1016/j.ejmech.2019.111700
- Sujatha, K., Shanthi, G., Selvam, N. P., Manoharan, S., Perumal, P. T., Rajendran, M. (2009). Synthesis and antiviral activity of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) against peste des petits ruminant virus (PPRV). Bioorganic & Medicinal Chemistry Letters, 19 (15), 4501–4503. https://doi.org/10.1016/j.bmcl.2009.02.113
- Farag, A. M., Mayhoub, A. S., Barakat, S. E., Bayomi, A. H. (2008). Regioselective synthesis and antitumor screening of some novel N-phenylpyrazole derivatives. Bioorganic & Medicinal Chemistry, 16 (2), 881–889. https://doi.org/10.1016/j.bmc.2007.10.015
- Dai, H., Ge, S., Guo, J., Chen, S., Huang, M., Yang, J., Sun, S., Ling, Y., Shi, Y. (2018). Development of novel bis-pyrazole derivatives as antitumor agents with potent apoptosis induction effects and DNA damage. European Journal of Medicinal Chemistry, 143, 1066–1076. https://doi.org/10.1016/j.ejmech.2017.11.098
- Cannarile, M. A., Weisser, M., Jacob, W., Jegg, A.-M., Ries, C. H., Rüttinger, D. (2017). Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. Journal for ImmunoTherapy of Cancer, 5 (1). https://doi.org/10.1186/s40425-017-0257-y
- Vanaparthi, S., Bantu, R., Jain, N., Janardhan, S., Nagarapu, L. (2020). Synthesis and anti-proliferative activity of a novel 1,2,3-triazole tethered chalcone acetamide derivatives. Bioorganic & Medicinal Chemistry Letters, 30 (16), 127304. https://doi.org/10.1016/j.bmcl.2020.127304
- Deville, S. S., Delgadillo Silva, L. F., Vehlow, A., Cordes, N. (2020). c-Abl Tyrosine Kinase Is Regulated Downstream of the Cytoskeletal Protein Synemin in Head and Neck Squamous Cell Carcinoma Radioresistance and DNA Repair. International Journal of Molecular Sciences, 21 (19), 7277. https://doi.org/10.3390/ijms21197277
- Naik, S., Soumya, V., Mamledesai, S. N., Manickavasagam, M., Choudhari, P., Rathod, S. (2024). Discovery of Substituted 2-oxoquinolinylthiazolidin-4-one Analogues as Potential EGFRK Inhibitors in Lung Cancer Treatment. Drug Research, 74 (5), 227–240. https://doi.org/10.1055/a-2305-2789
- Wu, B., Mao, Z. J., Wang, Z., Wu, P., Huang, H., Zhao, W., Zhang, L. et al. (2021). Deoxycytidine Kinase (DCK) Mutations in Human Acute Myeloid Leukemia Resistant to Cytarabine. Acta Haematologica, 144 (5), 534–541. https://doi.org/10.1159/000513696
- Drapak, I., Zimenkovsky, B., Perekhoda, L., Suleyman, М., Yeromina, H., Skaletska, N. et al. (2019). Search for angiotensin II receptor antagonists among 4-aryl-n-(aryl)-3-(prop-2-en-1-yl)-2,3-dihydro-1,3-thiazol-2-imine derivatives. Pharmacia, 66(4), 181–186. https://doi.org/10.3897/pharmacia.66.e36808
- Campobasso, N. (2019). C-abl Kinase domain with the activator(cmpd6), 2-cyano-N-(4-(3,4-dichlorophenyl)thiazol-2-yl)acetamide. RCSP PDB. https://doi.org/10.2210/pdb6npe/pdb
- Campobasso, N. (2019). C-abl Kinase domain with the activator(cmpd29), N-(1-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-3-yl)acetamide. RCSP PDB. https://doi.org/10.2210/pdb6npu/pdb
- Campobasso, N. (2019). C-abl Kinase domain with the activator(cmpd51), N-(1-(3,4-dichlorophenyl)-4-(2-hydroxyethyl)-4,5-dihydro-1H-pyrazol-3-yl)isonicotinamide. RCSP PDB. https://doi.org/10.2210/pdb6npv/pdb
- Saez-Ayala, M., Rebuffet, E., Hammam, K., Gros, L., Lopez, S., Hajem, B. et al. (2017). Crystal structure of dCK mutant C3S in complex with imatinib and UDP. RCSP PDB. https://doi.org/10.2210/pdb5mqt/pdb
- Zhang, Y., Zhang, C. (2015). Crystal structure of FMS kinase domain with a small molecular inhibitor, GLEEVEC. RCSP PDB. https://doi.org/10.2210/pdb4r7i/pdb
- Tap, W. D., Wainberg, Z. A., Anthony, S. P., Ibrahim, P. N., Zhang, C., Healey, J. H. et al. (2015). Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. New England Journal of Medicine, 373 (5), 428–437. https://doi.org/10.1056/nejmoa1411366
- Stamos, J., Sliwkowski, M. X., Eigenbrot, C. (2002). Structure of the Epidermal Growth Factor Receptor Kinase Domain Alone and in Complex with a 4-Anilinoquinazoline Inhibitor. Journal of Biological Chemistry, 277 (48), 46265–46272. https://doi.org/10.1074/jbc.m207135200
- Wibowo, A. S., Dann III, C. E. (2013). Human folate receptor beta (FOLR2) in complex with antifolate pemetrexed. RCSP PDB. https://doi.org/10.2210/pdb4kn2/pdb
- Wibowo, A. S., Singh, M., Reeder, K. M., Carter, J. J., Kovach, A. R., Meng, W. et al. (2013). Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proceedings of the National Academy of Sciences, 110 (38), 15180–15188. https://doi.org/10.1073/pnas.1308827110
- Fletcher, J. T., Christensen, J. A., Villa, E. M. (2017). Tandem synthesis of 1-formyl-1,2,3-triazoles. Tetrahedron Letters, 58 (47), 4450–4454. https://doi.org/10.1016/j.tetlet.2017.10.023
- Konovalova, I. S., Geleverya, A. O., Semenets, A., Kovalenko, S. M., Reiss, G. J. (2024). Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O. Zeitschrift Für Kristallographie - New Crystal Structures, 239 (5), 877–880. https://doi.org/10.1515/ncrs-2024-0213
- Danne, A. B., Deshpande, M. V., Sangshetti, J. N., Khedkar, V. M., Shingate, B. B. (2021). New 1,2,3-Triazole-Appended Bis-pyrazoles: Synthesis, Bioevaluation, and Molecular Docking. ACS Omega, 6 (38), 24879–24890. https://doi.org/10.1021/acsomega.1c03734
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Anna Geleverya, Anton Semenets, Sergiy М. Kovalenko, Marharyta Suleiman, Illya Podolsky, Lina Perekhoda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






