Problems of classification, safety assessment and risk management of medical devices with biologically active substances
DOI:
https://doi.org/10.15587/2519-4852.2025.328031Keywords:
medical devices, biologically active substances, biocompatibility assessment, medical device classification, regulatory requirements, ISO 10993, Regulation (EU) 2017/745, risk management, safety assessment methods, preclinical studies, regulatory framework harmonizationAbstract
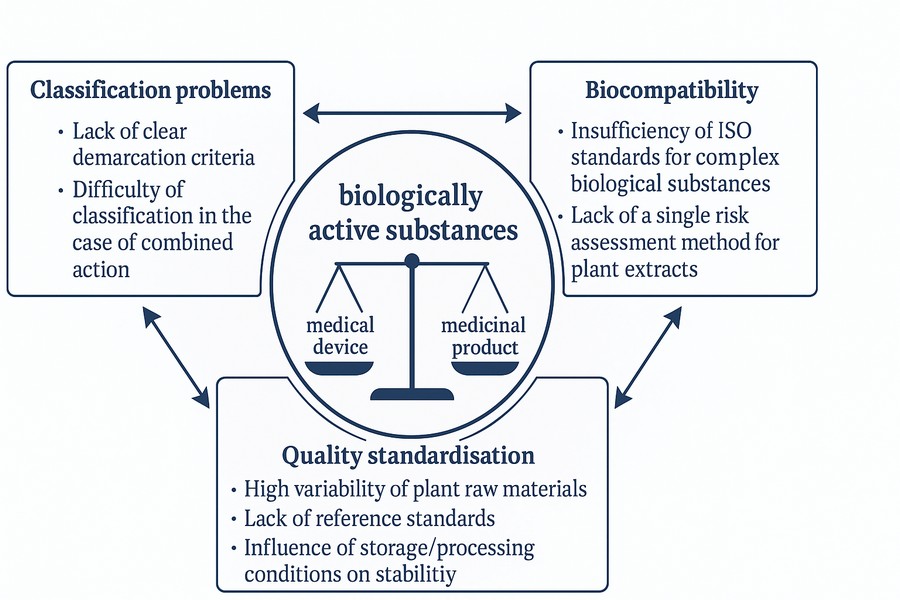
The aim of the work was to investigate scientific and technical approaches for ensuring biological safety and classifying medical devices containing biologically active substances in the context of new international standards and regulatory requirements.
Materials and methods. The study includes an analysis of the current regulatory landscape, including Regulation (EU) 2017/745, international standards ISO 10993, and guidance documents from the Medical Device Coordination Group (MDCG). Classification specificities of medical devices containing biologically active substances were examined, taking into account their mechanism of action. Approaches to biocompatibility assessment and systemic risk analysis were reviewed using the Weight of Evidence (WoE) concept.
Results. Key differences between the classification of medical devices under Directive 93/42/EEC and Regulation (EU) 2017/745 were analysed. The study identified specific aspects of biocompatibility assessment for medical devices containing biologically active substances, as well as challenges related to the standardisation of their physicochemical characteristics. Examples were provided to distinguish between medicinal products and medical devices based on their mechanism of action. The implementation of modern methods for assessing medical devices' safety and regulatory status was also examined.
Conclusions. The new regulatory requirements, in particular the provisions of Regulation (EU) 2017/745, significantly impact the classification and biological safety assessment of medical devices containing biologically active substances. An adaptation of the biocompatibility assessment methods was proposed, considering modern trends and the "Weight of Evidence" approach. The study highlights the need to update Ukraine's national regulatory framework to align with European standards and to introduce clearer methodological approaches to differentiate between medicinal products and medical devices
Supporting Agency
- Ministry of Education and Science of Ukraine grant “Development of a gel with wound healing properties for external use” (state registration number 0123U104137)
References
- Antich-Isern, P., Caro-Barri, J., Aparicio-Blanco, J. (2021). The combination of medical devices and medicinal products revisited from the new European legal framework. International Journal of Pharmaceutics, 607, 120992. https://doi.org/10.1016/j.ijpharm.2021.120992
- Bernard, M., Jubeli, E., Pungente, M. D., Yagoubi, N. (2018). Biocompatibility of polymer-based biomaterials and medical devices – regulations,in vitroscreening and risk-management. Biomaterials Science, 6 (8), 2025–2053. https://doi.org/10.1039/c8bm00518d
- European Economic Community. Council Directive 93/42/EEC of 14 of June 1993 concerning medical devices (1993). Official Journal of the European Communities, L 169/1. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31993L0042&from=EN
- European Union. Regulation (EU) No 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/43/EEC (2017). Official Journal of the European Communities, L 117. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745&from=EN
- Guidance on classification of medical devices (2021). Medical Device Coordination Group Document, MDCG 2021-24.
- Guidance on borderline between medical devices and medicinal products under Regulation (EU) 2017/745 on medical devices (2024). Medical Device Coordination Group Document, MDCG 2022-5, Rev 1.
- Pro zatverdzhennia Tekhnichnoho rehlamentu shchodo medychnykh vyrobiv (2013). Postanova Kabinet ministriv Ukrainy No. 753. 02.10.2013. Available from: https://zakon.rada.gov.ua/laws/show/753-2013-%D0%BF/page
- Pro zatverdzhennia metodychnykh rekomendatsii iz zastosuvannia Tekhnichnoho rehlamentu shchodo medychnykh vyrobiv, zatverdzhenoho postanovoiu Kabinetu Ministriv Ukrainy vid 02 zhovtnia 2013 roku No. 753, Tekhnichnoho rehlamentu shchodo medychnykh vyrobiv dlia diahnostyky in vitro, zatverdzhenoho postanovoiu Kabinetu Ministriv Ukrainy vid 02 zhovtnia 2013 roku No. 754, ta Tekhnichnoho rehlamentu shchodo aktyvnykh medychnykh vyrobiv, yaki implantuiut, zatverdzhenoho postanovoiu Kabinetu Ministriv Ukrainy vid 02 zhovtnia 2013 roku No. 755 (2020). Nakazom Ministerstva okhorony zdorovia No. 142. 22.01.2020. Available at: https://zakon.rada.gov.ua/rada/show/v0142282-20
- Pro likarski zasoby (2022). Zakon Ukrainy No. 2469-IX. 28.07.2022. Available at: https://zakon.rada.gov.ua/laws/show/2469-20
- Zinchenko, V., Chetverikov, S., Akhmad, E., Arzamasov, K., Vladzymyrskyy, A., Andreychenko, A., Morozov, S. (2022). Changes in software as a medical device based on artificial intelligence technologies. International Journal of Computer Assisted Radiology and Surgery, 17 (10), 1969–1977. https://doi.org/10.1007/s11548-022-02669-1
- Application of ISO/IEC 17021-1 in the Field of Medical Device Quality Management Systems (ISO 13485) (2023). IAF Mandatory Document.
- Malvehy, J., Ginsberg, R., Sampietro‐Colom, L., Ficapal, J., Combalia, M., Svedenhag, P. (2021). New regulation of medical devices in the EU: impact in dermatology. Journal of the European Academy of Dermatology and Venereology, 36 (3), 360–364. https://doi.org/10.1111/jdv.17830
- Martelli, N., Eskenazy, D., Déan, C., Pineau, J., Prognon, P., Chatellier, G. et al. (2019). New European Regulation for Medical Devices: What Is Changing? CardioVascular and Interventional Radiology, 42 (9), 1272–1278. https://doi.org/10.1007/s00270-019-02247-0
- Stevovic, J. (2019). What MDR class is my eHealth app? The Chino.io Blog. Available at: https://blog.chino.io/what-mdr-class-is-my-software/
- BSI Compliance Navigator. Available at: https://compliancenavigator.bsigroup.com/
- Melvin, T., Torre, M. (2019). New medical device regulations: the regulator’s view. EFORT Open Reviews, 4 (6), 351–356. https://doi.org/10.1302/2058-5241.4.180061
- Ludvigsen, K., Nagaraja, S., Daly, A. (2021). When Is Software a Medical Device? Understanding and Determining the “Intention” and Requirements for Software as a Medical Device in European Union Law. European Journal of Risk Regulation, 13 (1), 78–93. https://doi.org/10.1017/err.2021.45
- Liu, A.-A., Wang, Z.-G., Pang, D.-W. (2023). Medical Nanomaterials. Nanomedicine, 51–98. https://doi.org/10.1007/978-981-16-8984-0_5
- Chowdhury, N. (2012). Limits to the legal deliberation of science questions: A case study of borderline medical products in Europe. Pharmaceuticals, Policy and Law, 14 (2-4), 157–175. https://doi.org/10.3233/ppl-120351
- Tseliou, T. (2015). Balancing Protection of Public Health and Safety with the Free Movement of Goods in the EU Medical Device Sector: The Case of Borderline Productss Classification. SSRN Electronic Journal. https://doi.org/10.2139/ssrn.2585539
- Marletta, M. (2020). The new regulation 2017/745: an opportunity for innovation. Pharmadvances, 1 (1). https://doi.org/10.36118/pharmadvances.01.2020.03s
- Peter, L., Hajek, L., Maresova, P., Augustynek, M., Penhaker, M. (2020). Medical Devices: Regulation, Risk Classification, and Open Innovation. Journal of Open Innovation: Technology, Market, and Complexity, 6 (2), 42. https://doi.org/10.3390/joitmc6020042
- Morán, J., Kilasoniya, A. (2024). Viable Biological Materials or Organisms in Regulation (EU) 2017/745 on Medical Devices. https://doi.org/10.20944/preprints202407.0761.v1
- Leone, M. G. (2022). Medical Devices Made of Substances: A New Challenge. Frontiers in Drug Safety and Regulation, 2. https://doi.org/10.3389/fdsfr.2022.952013
- Bilia, A. R., Corazziari, E. S., Govoni, S., Mugelli, A., Racchi, M. (2021). Medical Devices Made of Substances: Possible Innovation and Opportunities for Complex Natural Products. Planta Medica, 87 (12/13), 1110–1116. https://doi.org/10.1055/a-1511-8558
- Racchi, M., Govoni, S., Lucchelli, A., Capone, L., Giovagnoni, E. (2016). Insights into the definition of terms in European medical device regulation. Expert Review of Medical Devices, 13 (10), 907–917. https://doi.org/10.1080/17434440.2016.1224644
- Manellari, S., Leone, M. G., Casiraghi, A., Gennari, C. G. M., Minghetti, P. (2022). Medicinal products, medical devices, or accessories of medical devices: How to qualify gases for spirometry? Frontiers in Drug Safety and Regulation, 2. https://doi.org/10.3389/fdsfr.2022.1089965
- Racchi, M., Govoni, S. (2020). The concept of non-pharmacological mechanism of action in medical devices made of substances in practice: what pharmacology can do to promote the scientific implementation of the European medical device regulation. Pharmadvances, 1 (1). https://doi.org/10.36118/pharmadvances.01.2020.02s
- MEDDEV 2.1/3 rev. 3 Borderline products, drug-delivery products and medical devices incorporating, as integral part, an ancillary medicinal substance or an ancillary human blood derivative. Available at: https://ec.europa.eu/docsroom/documents/10328/attachments/1/translations
- European Community. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal of the European Communities, L 311, 67. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32001L0083&from=en
- Sardi, C., Garetto, S., Capone, L., Galbiati, V., Racchi, M., Govoni, S. et al. (2018). Experimental Paradigm for the Assessment of the Non-pharmacological Mechanism of Action in Medical Device Classification: The Example of Glycerine as Laxative. Frontiers in Pharmacology, 9. https://doi.org/10.3389/fphar.2018.01410
- Santos, I. C., Gazelle, G. S., Rocha, L. A., Tavares, J. M. R. (2012). Medical device specificities: opportunities for a dedicated product development methodology. Expert Review of Medical Devices, 9 (3), 299–311. https://doi.org/10.1586/erd.12.3
- Fimognari, C., Barrajón-Catalán, E., Luceri, C., Turrini, E., Raschi, E., Bigagli, E. (2022). New regulation on medical devices made of substances: Opportunities and challenges for pharmacological and toxicological research. Frontiers in Drug Safety and Regulation, 2. https://doi.org/10.3389/fdsfr.2022.1001614
- Santus, P., Signorello, J. C., Danzo, F., Lazzaroni, G., Saad, M., Radovanovic, D. (2024). Anti-Inflammatory and Anti-Oxidant Properties of N-Acetylcysteine: A Fresh Perspective. Journal of Clinical Medicine, 13 (14), 4127. https://doi.org/10.3390/jcm13144127
- Pedre, B., Barayeu, U., Ezeriņa, D., Dick, T. P. (2021). The mechanism of action of N-acetylcysteine (NAC): The emerging role of H2S and sulfane sulfur species. Pharmacology & Therapeutics, 228, 107916. https://doi.org/10.1016/j.pharmthera.2021.107916
- Public data from Article 57 database. European Medicines Agency (EMA). Available at: https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/data-medicines-iso-idmp-standards-post-authorisation/public-data-article-57-database
- Rinofast Flu. PJPHARMA. Available at: https://www.pjpharma.it/en/products/rinofast-flu
- Viscoflu linea. pharma-line.it Available at: https://pharma-line.it/en/prodotto-exp/viscoflu-linea/
- Devices/Systems/Procedure packs. EUDAMED. Available at: https://ec.europa.eu/tools/eudamed/#/screen/search-device?tradeName=Vagi-C%C2%AE&deviceStatusCode=refdata.device-model-status.on-the-market&submitted=true
- Meloni, M., De Servi, B., Carriero, F., Simon O’Brien, E., Houamel, D., Deruelle, P., Castagné, V. (2024). Demonstrating the principal mechanism of action of medical devices intended for vaginal use on reconstructed human vaginal epithelium: the case of two hyaluronic acid-containing devices. Frontiers in Drug Safety and Regulation, 4. https://doi.org/10.3389/fdsfr.2024.1445519
- Huang, T., Zhang, Y., Zhao, L., Ren, Y., Wang, K., Zhang, N. et al. (2024). Sodium hyaluronate hydrogel for wound healing and human health monitoring based on deep eutectic solvent. International Journal of Biological Macromolecules, 257, 128801. https://doi.org/10.1016/j.ijbiomac.2023.128801
- Yasin, A., Ren, Y., Li, J., Sheng, Y., Cao, C., Zhang, K. (2022). Advances in Hyaluronic Acid for Biomedical Applications. Frontiers in Bioengineering and Biotechnology, 10. https://doi.org/10.3389/fbioe.2022.910290
- Vasvani, S., Kulkarni, P., Rawtani, D. (2020). Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. International Journal of Biological Macromolecules, 151, 1012–1029. https://doi.org/10.1016/j.ijbiomac.2019.11.066
- Juncan, A. M., Moisă, D. G., Santini, A., Morgovan, C., Rus, L.-L., Vonica-Țincu, A. L., Loghin, F. (2021). Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules, 26 (15), 4429. https://doi.org/10.3390/molecules26154429
- Tamer, T. M. (2013). Hyaluronan and synovial joint: function, distribution and healing. Interdisciplinary Toxicology, 6 (3), 111–125. https://doi.org/10.2478/intox-2013-0019
- Vassallo, V., Di Meo, C., Toro, G., Alfano, A., Iolascon, G., Schiraldi, C. (2023). Hyaluronic Acid-Based Injective Medical Devices: In Vitro Characterization of Novel Formulations Containing Biofermentative Unsulfated Chondroitin or Extractive Sulfated One with Cyclodextrins. Pharmaceuticals, 16 (10), 1429. https://doi.org/10.3390/ph16101429
- Gupta, R. C., Lall, R., Srivastava, A., Sinha, A. (2019). Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Frontiers in Veterinary Science, 6. https://doi.org/10.3389/fvets.2019.00192
- Rah, M. J. (2011). A review of hyaluronan and its ophthalmic applications. Optometry – Journal of the American Optometric Association, 82 (1), 38–43. https://doi.org/10.1016/j.optm.2010.08.003
- Marchesi, N., Fahmideh, F., Barbieri, A., Racchi, M., Pascale, A., Govoni, S. (2022). Pharmacological Versus Non-Pharmacological and Ancillary Mechanisms in Eye Drops Used in the Treatment of Glaucoma. Frontiers in Drug Safety and Regulation, 2. https://doi.org/10.3389/fdsfr.2022.933471
- Jasielski, P., Piędel, F., Piwek, M., Rocka, A., Petit, V., Rejdak, K. (2020). Application of Citicoline in Neurological Disorders: A Systematic Review. Nutrients, 12(10), 3113. https://doi.org/10.3390/nu12103113
- Świątkiewicz, M., Grieb, P. (2022). Citicoline for Supporting Memory in Aging Humans. Aging and Disease, 14 (4), 1184–1195. https://doi.org/10.14336/ad.2022.0913
- Grieb, P. (2015). Citicoline: A Food That May Improve Memory. Medical Science Review, 2, 67–72. https://doi.org/10.12659/msrev.894711
- Gandolfi, S., Marchini, G., Caporossi, A., Scuderi, G., Tomasso, L., Brunoro, A. (2020). Cytidine 5′-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients, 12 (3), 793. https://doi.org/10.3390/nu12030793
- Faiq, M. A., Wollstein, G., Schuman, J. S., Chan, K. C. (2019). Cholinergic nervous system and glaucoma: From basic science to clinical applications. Progress in Retinal and Eye Research, 72, 100767. https://doi.org/10.1016/j.preteyeres.2019.06.003
- Carnevale, C., Manni, G., Roberti, G., Micera, A., Bruno, L., Cacciamani, A. et al. (2019). Human vitreous concentrations of citicoline following topical application of citicoline 2% ophthalmic solution. PLOS ONE, 14 (11), e0224982. https://doi.org/10.1371/journal.pone.0224982
- OMK1 Citicoline Eye Drop. Omikron. Available at: https://www.citicolineomk1.com/
- Gilbert, B., Alves, L. (2003). Synergy in Plant Medicines. Current Medicinal Chemistry, 10 (1), 13–20. https://doi.org/10.2174/0929867033368583
- Thomford, N. E., Senthebane, D. A., Rowe, A., Munro, D., Seele, P., Maroyi, A., Dzobo, K. (2018). Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. International Journal of Molecular Sciences, 19 (6), 1578. https://doi.org/10.3390/ijms19061578
- Wanjek, C. (2022). Systems Biology as defined by NIH. NIH Intramural Research Program. Available at: https://irp.nih.gov/catalyst/19/6/systems-biology-as-defined-by-nih
- Giovagnoni, E. (2022). Substance-based medical devices made of natural substances: An opportunity for therapeutic innovation. Frontiers in Drug Safety and Regulation, 2. https://doi.org/10.3389/fdsfr.2022.998114
- Guarino, G., Della Corte, T., Strollo, F., Gentile, S. (2021). Policaptil Gel Retard in adult subjects with the metabolic syndrome: Efficacy, safety, and tolerability compared to metformin. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 15 (3), 901–907. https://doi.org/10.1016/j.dsx.2021.03.032
- Mercati, V. (2005). EP1679009A1. Pharmaceutical and dietetic compositions based on vegetable fibres. Available at: https://patents.google.com/patent/EP1679009A1/en
- Greco, C. M., Garetto, S., Montellier, E., Liu, Y., Chen, S., Baldi, P. et al. (2020). A non-pharmacological therapeutic approach in the gut triggers distal metabolic rewiring capable of ameliorating diet-induced dysfunctions encompassed by metabolic syndrome. Scientific Reports, 10 (1). https://doi.org/10.1038/s41598-020-69469-y
- Stagi, S., Lapi, E., Seminara, S., Pelosi, P., Del Greco, P., Capirchio, L. et al. (2015). Policaptil Gel Retard® significantly reduces body mass index and hyperinsulinism and may decrease the risk of type 2 diabetes mellitus (T2DM) in obese children and adolescents with family history of obesity and T2DM. Italian Journal of Pediatrics, 41 (1). https://doi.org/10.1186/s13052-015-0109-7
- Guarino, G., Strollo, F., Della-Corte, T., Satta, E., Romano, C., Alfarone, C. et al. (2022). Comparison between Policaptil Gel Retard and Metformin by Testing of Temporal Changes in Patients with Metabolic Syndrome and Type 2 Diabetes. Diabetology, 3 (2), 315–327. https://doi.org/10.3390/diabetology3020022
- Marletta, M. (2024). Regulation 2017/745 on medical devices, two major innovations: 1) the physiological action of devices consisting of natural materials such as vegetal matrices; 2) the chemical-physical-mechanical action of devices made of “substances”, which as such are artificial derivatives. Frontiers in Drug Safety and Regulation, 4. https://doi.org/10.3389/fdsfr.2024.1389406
- Lee, S. (2015). Systems Biology – A Pivotal Research Methodology for Understanding the Mechanisms of Traditional Medicine. Journal of Pharmacopuncture, 18 (3), 11–18. https://doi.org/10.3831/kpi.2015.18.020
- Rai, S., Raj, U., Varadwaj, P. K. (2018). Systems Biology: A Powerful Tool for Drug Development. Current Topics in Medicinal Chemistry, 18 (20), 1745–1754. https://doi.org/10.2174/1568026618666181025113226
- Chaachouay, N., Zidane, L. (2024). Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs and Drug Candidates, 3 (1), 184–207. https://doi.org/10.3390/ddc3010011
- Aronson, J. K., Heneghan, C., Ferner, R. E. (2019). Medical Devices: Definition, Classification, and Regulatory Implications. Drug Safety, 43 (2), 83–93. https://doi.org/10.1007/s40264-019-00878-3
- Gorchakova, N., Harnyk, T., Khudetskyy, I., Dmytrenko, A., Bespalova, O., Biloshytska, O. (2024). Analysis of current data on the safety and efficacy of the phloroglucinol and simeticone combination (literature review). Fitoterapia, 2, 5–20. https://doi.org/10.32782/2522-9680-2024-2-5
- De Jong, W. H., Carraway, J. W., Geertsma, R. E. (2012). In vivo and in vitro testing for the biological safety evaluation of biomaterials and medical devices. Biocompatibility and Performance of Medical Devices, 120–158. https://doi.org/10.1533/9780857096456.2.120
- Kanďárová, H., Pôbiš, P. (2024). The “Big Three” in biocompatibility testing of medical devices: implementation of alternatives to animal experimentation –are we there yet? Frontiers in Toxicology, 5. https://doi.org/10.3389/ftox.2023.1337468
- ISO 10993-1:2018 (2018). Biological evaluation of medical devices. Part 1: evaluation and testing within a risk management process. Edition 5. Geneva: International Organization for Standardization.
- Sündermann, J., Bitsch, A., Kellner, R., Doll, T. (2024). Is read-across for chemicals comparable to medical device equivalence and where to use it for conformity assessment? Regulatory Toxicology and Pharmacology, 149, 105622. https://doi.org/10.1016/j.yrtph.2024.105622
- Larionov, V., Golovenko, M., Valivodz, I., Reder, A. (2025). Inhibition of Cytochrome P450 Activities by Propoxazepam: Safety Assessment in Context for Potential Drug Interactions. Innovative Biosystems and Bioengineering, 9 (2), 4–11. https://doi.org/10.20535/ibb.2025.9.2.309378
- Skavinska, O. О., Rossokha, Z. I., Podolska, S. V., Ievseienkova, O. G., Buriak, O. A., Olkhovych, V. P., Gorovenko, N. G. (2024). The role of pharmacogenomic studies in increasing the effectiveness and safety of clinical application of statins. Clinical and Preventive Medicine, 5, 109–123. https://doi.org/10.31612/2616-4868.5.2024.14
- Halkin, O. Iu., Savchenko, A. A., Nikitina, K. I., Duhan, O. M. (1999). Isolation and characterization of new monoclonal antibodies against human IgE. Ukrainian Biochemical Journal, 85 (5), 81–87. Available at: https://pubmed.ncbi.nlm.nih.gov/24479325/
- Shevchuk, K., Baranovska, A., Chernetskyi, A., Besarab, A. (2025). Biosafety Aspects of Hybridoma Technology: Nature of Risks and Approaches to their Management. Innovative Biosystems and Bioengineering, 9 (2), 29–41. https://doi.org/10.20535/ibb.2025.9.2.320712
- Street, S. M., Christian, W. V. (2024). Taring the scales: Weight-of-evidence framework for biocompatibility evaluations. Regulatory Toxicology and Pharmacology, 149, 105590. https://doi.org/10.1016/j.yrtph.2024.105590
- ISO 10993-5:2009 (2009). Biological evaluation of medical devices. Part 5: tests for in vitro cytotoxicity. Edition 3. Geneva: International Organization for Standardization.
- ISO 10993-10:2021 (2021). Biological evaluation of medical devices. Part 10: tests for skin sensitization. Edition 4. Geneva: International Organization for Standardization.
- ISO 10993-4:2017 (2017). Biological evaluation of medical devices. Part 4: selection of tests for interactions with blood. Edition 3. Geneva: International Organization for Standardization.
- ISO 10993-11:2017 (2017). Biological evaluation of medical devices. Part 11: tests for systemic toxicity. Edition 3. Geneva: International Organization for Standardization.
- Fomina, N. S., Kovalchuk, V. P., Vovk, I. M., Fomin, O. O., Kovalenko, I. M. (2024). Antimicrobial activity assessment of food preservatives containing organic carboxylic acids. Clinical and Preventive Medicine, 5, 80–86. https://doi.org/10.31612/2616-4868.5.2024.10
- Key learnings from ISO 10993-23:2021: Biological evaluation of medical devices – Tests for irritation. Congenius. 2022. Available at: https://congenius.ch/biological-evaluation-medical-devices-tests-for-irritation/
- Gruber, S., Nickel, A. (2023). Toxic or not toxic? The specifications of the standard ISO 10993-5 are not explicit enough to yield comparable results in the cytotoxicity assessment of an identical medical device. Frontiers in Medical Technology, 5. https://doi.org/10.3389/fmedt.2023.1195529
- Stordeur, S., Vinck, I., Neyt, M., Van Brabandt, H., Hulstaert, F. (2013). Mise sur le marché européen des dispositifs médicaux innovants à haut risque : l’efficacité clinique et la sécurité sont-elles garanties ? Revue d’Épidémiologie et de Santé Publique, 61 (2), 105–110. https://doi.org/10.1016/j.respe.2012.08.004
- Räägel, H., Turley, A., Fish, T., Franson, J., Rollins, T., Campbell, S., Jorgensen, M. R. (2021). Medical Device Industry Approaches for Addressing Sources of Failing Cytotoxicity Scores. Biomedical Instrumentation & Technology, 55 (2), 69–84. https://doi.org/10.2345/0899-8205-55.2.69
- Goller, S., Turner, N. J. (2020). The Antimicrobial Effectiveness and Cytotoxicity of the Antibiotic-Loaded Chitosan: ECM Scaffolds. Applied Sciences, 10 (10), 3446. https://doi.org/10.3390/app10103446
- Suter, G., Cormier, S., Barron, M. (2017). A weight of evidence framework for environmental assessments: Inferring qualities. Integrated Environmental Assessment and Management, 13 (6), 1038–1044. https://doi.org/10.1002/ieam.1954
- Suter, G., Cormier, S., Barron, M. (2017). A weight of evidence framework for environmental assessments: Inferring quantities. Integrated Environmental Assessment and Management, 13 (6), 1045–1051. https://doi.org/10.1002/ieam.1953
- Pistollato, F., Madia, F., Corvi, R., Munn, S., Grignard, E., Paini, A. et al. (2021). Current EU regulatory requirements for the assessment of chemicals and cosmetic products: challenges and opportunities for introducing new approach methodologies. Archives of Toxicology, 95 (6), 1867–1897. https://doi.org/10.1007/s00204-021-03034-y
- Rocca, M., Morford, L. L., Blanset, D. L., Halpern, W. G., Cavagnaro, J., Bowman, C. J. (2018). Applying a weight of evidence approach to the evaluation of developmental toxicity of biopharmaceuticals. Regulatory Toxicology and Pharmacology, 98, 69–79. https://doi.org/10.1016/j.yrtph.2018.07.006
- Dmytrenko, O., Lutsenko, T., Dmytrenko, A., Bespalova, O. (2024). Assessment of Efficiency and Safety of Phytocomposition with Prostate-Protective Properties in the form of Rectal Suppositories. Natural and Engineering Sciences, 9 (2), 407–425. https://doi.org/10.28978/nesciences.1465276
- Dronko, L. M., Lutsenko, T. M., Korotkevych, N. V., Vovk, I. O., Zhukova, D. A., Romaniuk, S. I. et al. (2024). Heparin-binding EGF-like growth factor: mechanisms of biological activity and potential therapeutic applications. The Ukrainian Biochemical Journal, 96 (5), 5–20. https://doi.org/10.15407/ubj96.05.005
- Strickland, J., Haugabrooks, E., Allen, D. G., Balottin, L. B., Hirabayashi, Y., Kleinstreuer, N. C. et al. (2023). International regulatory uses of acute systemic toxicity data and integration of new approach methodologies. Critical Reviews in Toxicology, 53 (7), 385–411. https://doi.org/10.1080/10408444.2023.2240852
- Tandy, J., Hanhquynh Le, K., Michael Deane, G., Joseph Burns, S. (2022). Cleanability of Metal Surface Finishes Found in Medical Devices and the Environment of Care. Biomedical Instrumentation & Technology, 56 (2), 29–36. https://doi.org/10.2345/1943-5967-56.2.29
- Sussman, E. M., Oktem, B., Isayeva, I. S., Liu, J., Wickramasekara, S., Chandrasekar, V. et al. (2022). Chemical Characterization and Non-targeted Analysis of Medical Device Extracts: A Review of Current Approaches, Gaps, and Emerging Practices. ACS Biomaterials Science & Engineering, 8 (3), 939–963. https://doi.org/10.1021/acsbiomaterials.1c01119
- ISO 10993-17:2023 (2023). Biological evaluation of medical devices. Part 17: toxicological risk assessment of medical device constituents. Edition 2. Geneva: International Organization for Standardization.
- Toxicological Risk Assessment of Medical Devices. Available at: https://www.tuvsud.com/en/industries/healthcare-and-medical-devices/medical-devices-and-ivd/medical-device-testing/toxicological-risk-assessment-of-medical-devices
- ISO 10993-18:2020 (2020). Biological evaluation of medical devices. Part 18: chemical characterization of medical device materials within a risk management process. Edition 2. Geneva: International Organization for Standardization.
- ISO/TS 10993-19:2020 (2020). Biological evaluation of medical devices. Part 19: physico-chemical, morphological and topographical characterization of materials. Edition 2. Geneva: International Organization for Standardization.
- Liu, X., Rodeheaver, D. P., White, J. C., Wright, A. M., Walker, L. M., Zhang, F., Shannon, S. (2018). A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regulatory Toxicology and Pharmacology, 97, 24–32. https://doi.org/10.1016/j.yrtph.2018.06.003
- McDermott, O., Kearney, B. (2023). The value of using real-world evidence as a source of clinical evidence in the European medical device regulations: a mixed methods study. Expert Review of Medical Devices, 21 (1-2), 149–163. https://doi.org/10.1080/17434440.2023.2291454
- Pane, J., Francisca, R. D. C., Verhamme, K. M. C., Orozco, M., Viroux, H., Rebollo, I., Sturkenboom, M. C. J. M. (2019). EU postmarket surveillance plans for medical devices. Pharmacoepidemiology and Drug Safety, 28 (9), 1155–1165. https://doi.org/10.1002/pds.4859
- Establishing quality specifications for medicines, vaccines and in vitro diagnostics: week of quality 2023 training kit (2024). World Health Organization. Available at: https://www.who.int/publications/i/item/978924009599
- Albert, D. E. (2012). Material and chemical characterization for the biological evaluation of medical device biocompatibility. Biocompatibility and Performance of Medical Devices. Elsevier eBooks, 65–94. https://doi.org/10.1533/9780857096456.2.63
- Kramer D. B., Tan, Y. T., Sato, C., Kesselheim, A. S. (2014). Ensuring medical device effectiveness and safety: a cross--national comparison of approaches to regulation. Food and Drug Law Journal, 69 (1), 1–23. Available at: https://pubmed.ncbi.nlm.nih.gov/24772683
- Galkin, O. Yu., Lutsenko, T. M., Gorshunov, Yu. V., Motronenko, V. V. (2017). Development of the method for microbiological purity testing of recombinant human interleukin-7-based product. The Ukrainian Biochemical Journal, 89(3), 52–59. https://doi.org/10.15407/ubj89.03.052
- Umamaheswari, D., Muthuraja, R., Kumar, M., Venkateswarlu, B. S. (2021). Standardization of Herbal Drugs – A Overview. International Journal of Pharmaceutical Sciences Review and Research, 68 (1). https://doi.org/10.47583/ijpsrr.2021.v68i01.033
- Noviana, E., Indrayanto, G., Rohman, A. (2022). Advances in Fingerprint Analysis for Standardization and Quality Control of Herbal Medicines. Frontiers in Pharmacology, 13. https://doi.org/10.3389/fphar.2022.853023
- Atanasov, A. G., Waltenberger, B., Pferschy-Wenzig, E.-M., Linder, T., Wawrosch, C., Uhrin, P. et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology Advances, 33 (8), 1582–1614. https://doi.org/10.1016/j.biotechadv.2015.08.001
- Bagade, S., Patil, D. D., Shirkhedkar, A. (2022). Standardization of herbal bioactives. Herbal Bioactive-Based Drug Delivery Systems. Elsevier eBooks, 393–407. https://doi.org/10.1016/b978-0-12-824385-5.00005-4
- Wang, H., Chen, Y., Wang, L., Liu, Q., Yang, S., Wang, C. (2023). Advancing herbal medicine: enhancing product quality and safety through robust quality control practices. Frontiers in Pharmacology, 14. https://doi.org/10.3389/fphar.2023.1265178
- Barba-Ostria, C., Carrera-Pacheco, S. E., Gonzalez-Pastor, R., Heredia-Moya, J., Mayorga-Ramos, A., Rodríguez-Pólit, C. et al. (2022). Evaluation of Biological Activity of Natural Compounds: Current Trends and Methods. Molecules, 27 (14), 4490. https://doi.org/10.3390/molecules27144490
- Govindaraghavan, S., Sucher, N. J. (2015). Quality assessment of medicinal herbs and their extracts: Criteria and prerequisites for consistent safety and efficacy of herbal medicines. Epilepsy & Behavior, 52, 363–371. https://doi.org/10.1016/j.yebeh.2015.03.004
- Li, Y., Kong, D., Fu, Y., Sussman, M. R., Wu, H. (2020). The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiology and Biochemistry, 148, 80–89. https://doi.org/10.1016/j.plaphy.2020.01.006
- Ponphaiboon, J., Krongrawa, W., Aung, W. W., Chinatangkul, N., Limmatvapirat, S., Limmatvapirat, C. (2023). Advances in Natural Product Extraction Techniques, Electrospun Fiber Fabrication, and the Integration of Experimental Design: A Comprehensive Review. Molecules, 28 (13), 5163. https://doi.org/10.3390/molecules28135163
- Khudetskyy, I., Antonova-Rafi, J. (2023). Human influence and changes in nature on biological security (overview of the problem). Fitoterapia, 2, 26–34. https://doi.org/10.32782/2522-9680-2023-2-37
- Klein-Junior, L. C., de Souza, M. R., Viaene, J., Bresolin, T. M. B., de Gasper, A. L., Henriques, A. T., Heyden, Y. V. (2021). Quality Control of Herbal Medicines: From Traditional Techniques to State-of-the-art Approaches. Planta Medica, 87 (12/13), 964–988. https://doi.org/10.1055/a-1529-8339
- Guidance on standardisation for medical devices (2024). MDCG 2021-5, Rev. 1. Medical Device Coordination Group.
- Golembiovska, O., Dmytrenko, O., Galkin, A. (2024). Design and Development of Novel Herbal Suppository Formulation for Prostatitis Treatment. Innovative Biosystems and Bioengineering, 8 (4), 23–38. https://doi.org/10.20535/ibb.2024.8.4.317124
- Görög, S. (2018). Critical review of reports on impurity and degradation product profiling in the last decade. TrAC Trends in Analytical Chemistry, 101, 2–16. https://doi.org/10.1016/j.trac.2017.09.012
- Singh, G., Lu, D., Liu, C., Hower, D. (2021). Analytical challenges and recent advances in the identification and quantitation of extractables and leachables in pharmaceutical and medical products. TrAC Trends in Analytical Chemistry, 141, 116286. https://doi.org/10.1016/j.trac.2021.116286
- Ram, M., Abdin, M. Z., Khan, M. A., Jha, P. (2010). HPTLC Fingerprint Analysis: A Quality Control for Authentication of Herbal Phytochemicals. High-Performance Thin-Layer Chromatography (HPTLC). Springer eBooks, 105–116. https://doi.org/10.1007/978-3-642-14025-9_7
- Sharma, A., Chauhan, R., Kumar, R., Mankotia, P., Verma, R., Sharma, V. (2021). A rapid and non-destructive ATR-FTIR spectroscopy method supported by chemometrics for discriminating between facial creams and the classification into herbal and non-herbal brands. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 258, 119803. https://doi.org/10.1016/j.saa.2021.119803
- Muyumba, N. W., Mutombo, S. C., Sheridan, H., Nachtergael, A., Duez, P. (2021). Quality control of herbal drugs and preparations: The methods of analysis, their relevance and applications. Talanta Open, 4, 100070. https://doi.org/10.1016/j.talo.2021.100070
- Indrayanto, G. (2022). The importance of method validation in herbal drug research. Journal of Pharmaceutical and Biomedical Analysis, 214, 114735. https://doi.org/10.1016/j.jpba.2022.114735
- Hasija, M., Sheung, A., Rahman, N., Ausar, S. F. (2016). Stressed Stability Techniques for Adjuvant Formulations. Vaccine Adjuvants, 227–238. https://doi.org/10.1007/978-1-4939-6445-1_16
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Oleksandra Dmytrenko, Olena Golembiovska, Viktoriia Pashuk, Svitlana Zgonnyk

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






