Study of the solubility of betamethasone dipropionate and the conditions for the formation of the stable suspensions
DOI:
https://doi.org/10.15587/2519-4852.2025.333181Keywords:
betamethasone dipropionate (BD), solubility, crystallization, propylene glycol (PG), water, solvent, liquid paraffinAbstract
The aim. To study the solubility of betamethasone dipropionate (BD) in mixed solvents water – propylene glycol (PG) and liquid paraffin, as well as to identify the conditions necessary for the formation of stable BD suspensions.
Materials and methods. The solubility of BD in solvents water – PG was studied using spectrophotometry. The particle size distribution of BD in suspensions was analysed by laser diffraction. The suspensions and creams were subjected to optical microscopy for additional evaluation. Thermogravimetric analysis was conducted to explore the potential formation of BD crystallosolvates with PG, while differential scanning calorimetry was utilised to assess the characteristics of the dissolution processes. Additionally, the study employed the spin probe method, using a steroid spin-label.
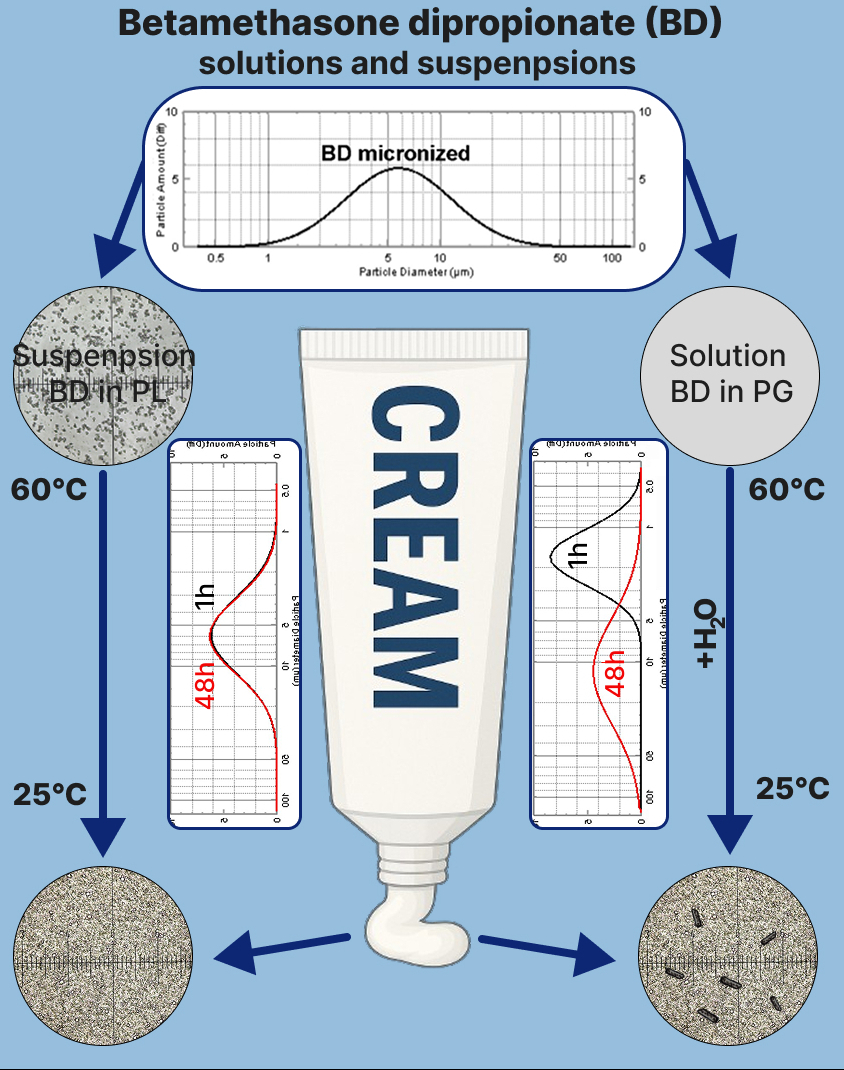
Results. The solubility of BD in water – PG solvents was found to increase with rising temperature and to increase sharply with increasing PG concentration, provided that the PG structure dominated the system. The deviations of BD solubility from additivity at 20-35°C were negative, passing through a minimum at a PG concentration of ~35% mol, above which the transition to the structure of a nonaqueous solvent occurred. An elevation in temperature to 45-55°C resulted in a positive deviation of the BD solubility from additive values at specific PG concentrations. It has been demonstrated that PG and BD do not form crystallosolvates. The process of dissolving BD in PG is exothermic, while in liquid paraffin, it is endothermic. The steroid spin probe was found to be localized in the oil phases of creams. Suspensions in which BD particles recrystallize were formed when BD crystallized from solution in PG as a result of lowering the temperature and adding water. When BD was suspended in a water ‒ PG solvent, where the water structure predominates, or in liquid paraffin (oil phase of creams), the BD particle size increased slightly, or there was no recrystallization.
Conclusions. The solubility of BD in solvents water – PG is contingent upon the temperature and concentration of PG; it exhibits a marked increase when the structure of a nonaqueous solvent predominates in the system. It has been demonstrated that BD with PG does not form crystallosolvates. When BD suspensions were obtained by crystallization from a solution in PG, suspensions were formed in which BD particles recrystallized over time. In the case of BD suspensions in solvent water – PG, where the water structure predominates, or in liquid paraffin, recrystallization was practically not observed
Supporting Agency
- The research was financially supported by the National Academy of Sciences of Ukraine within the framework of the project «Study of dispersed systems with liquid dispersion medium as the primary matrices for medicinal products» (0125U000740).
References
- The European Pharmacopoeia (2022). European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Strasbourg: Sedex, 6105. Available at: http://pheur.edqm.eu/subhome/11-0
- The United States Pharmacopoeia 46 ed. The National Formulary 41 [USP 46 – NF 41] (2023). The United States Pharmacopeial Convention. Rockville: United Book Press, Inc.
- British Pharmacopoeia (2025). London: The Stationery Office. Available at: https://www.pharmacopoeia.com/
- Buckingham, R. (Ed.) (2020). Martindale: The Complete Drug Reference, 40th Ed. London: Pharmaceutical Press, 4852.
- ATC/DDD Index (2025). WHO Collaborating Centre for Drug Statistics Methodology. Oslo: Norwegian Institute of Public Health.
- Derzhavnyi reiestr likarskykh zasobiv Ukrainy. Available at: http://www.drlz.kiev.ua/
- Sheskey, P. J., Hancock, B. C., Moss, G. P., Goldfarb, D. J. (Eds.) (2020). Handbook of Pharmaceutical Excipients. London: Pharm. Press, 1296.
- Bezuhlaia, E. P., Melnykova, E. N., Zhemerova, E. H., Liapunov, A. N., Zynchenko, Y. A. (2016). Efficacy of antimicrobial preservation of certain hydrophilic non-aqueous solvents in aqueous solutions and gels. Farmakom, 1, 51–59.
- Bezugla, O. P., Lyapunov, M. O., Zinchenko, I. O., Lisokobilka, O. A., Liapunova, A. M. (2022). Modeling of processes of solvent diffusion from ointment bases using in vitro experiments. Functional materials, 29 (4), 553–558. https://doi.org/10.15407/fm29.04.553
- Bendas, B., Schmalfuβ, U., Neubert, R. (1995). Influence of propylene glycol as cosolvent on mechanisms of drug transport from hydrogels. International Journal of Pharmaceutics, 116 (1), 19–30. https://doi.org/10.1016/0378-5173(94)00267-9
- Carrer, V., Alonso, C., Pont, M., Zanuy, M., Córdoba, M., Espinosa, S. et al. (2019). Effect of propylene glycol on the skin penetration of drugs. Archives of Dermatological Research, 312 (5), 337–352. https://doi.org/10.1007/s00403-019-02017-5
- Liapunova, A. M., Krasnopyorova, А. P., Bezuglа, О. P., Liapunov, O. M., Yukhnо, G. D., Pukhova, T. М. (2024). Polythermal studies of the water – propylene glycol systems by densitometry, viscometry and spin probes method. Functional Materials, 31 (4), 609–618. https://doi.org/10.15407/fm31.04.609
- Khattab, I. S., Bandarkar, F., Khoubnasabjafari, M., Jouyban, A. (2017). Density, viscosity, surface tension, and molar volume of propylene glycol + water mixtures from 293 to 323 K and correlations by the Jouyban–Acree model. Arabian Journal of Chemistry, 10, S71–S75. https://doi.org/10.1016/j.arabjc.2012.07.012
- Makarov, D. M., Egorov, G. I., Kolker, A. M. (2016). Temperature and composition dependences of volumetric properties of (water + 1,2-propanediol) binary system. Journal of Molecular Liquids, 222, 656–662. https://doi.org/10.1016/j.molliq.2016.07.095
- Jimeneze, J., Martinez, F. (2005). Study of some volumetric properties of 1,2-propanediol + water mixtures at several temperatures. Revista Colombiana de Ciencias Químico-Farmacéuticas, 34 (1), 46‒57.
- Sun, T., Teja, A. S. (2004). Density, Viscosity and Thermal Conductivity of Aqueous Solutions of Propylene Glycol, Dipropylene Glycol, and Tripropylene Glycol between 290 K and 460 K. Journal of Chemical & Engineering Data, 49 (5), 1311–1317. https://doi.org/10.1021/je049960h
- dos Santos, L. J., Espinoza-Velasquez, L. A., Coutinho, J. A. P., Monteiro, S. (2020). Theoretically consistent calculation of viscous activation parameters through the Eyring equation and their interpretation. Fluid Phase Equilibria, 522, 112774. https://doi.org/10.1016/j.fluid.2020.112774
- Xu, Y., Xing, L., Cao, X., Li, D., Men, Z., Li, Z. et al. (2023). Hydrogen bonding network dynamics of 1,2-propanediol-water binary solutions by Raman spectroscopy and stimulated Raman scattering. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 284, 121825. https://doi.org/10.1016/j.saa.2022.121825
- Zhou, Y., Hu, K., Shen, J., Wu, X., Cheng, G. (2009). Microstructure variations with concentration of propylene glycol–water solution probed by NMR. Journal of Molecular Structure, 921 (1-3), 150–155. https://doi.org/10.1016/j.molstruc.2008.12.050
- Panahi-Azar, V., Shayanfar, A., Martínez, F., Acree Jr, W. E., Jouyban, A. (2011). Thermodynamic studies of fluphenazine decanoate solubility in propylene glycol+water mixtures and correlation with the Jouyban-Acree model. Fluid Phase Equilibria, 308 (1-2), 72–77. https://doi.org/10.1016/j.fluid.2011.06.008
- Zeng, A.-G., Pang, X.-L., Wu, N., Wang, D., Nan, G.-J., Yang, G.-D., Bian, X.-L. (2014). Solubility of daidzein in propylene glycol plus water cosolvent mixtures. Fluid Phase Equilibria, 366, 127–133. https://doi.org/10.1016/j.fluid.2013.12.024
- Fathi-Azarjbayjani, A., Mabhoot, A., Martínez, F., Jouyban, A. (2016). Modeling, solubility, and thermodynamic aspects of sodium phenytoin in propylene glycol–water mixtures. Journal of Molecular Liquids, 219, 68–73. https://doi.org/10.1016/j.molliq.2016.02.089
- Jouyban-Gharamaleki, V., Rahimpour, E., Hemmati, S., Martinez, F., Jouyban, A. (2020). Mesalazine solubility in propylene glycol and water mixtures at various temperatures using a laser monitoring technique. Journal of Molecular Liquids, 299, 112136. https://doi.org/10.1016/j.molliq.2019.112136
- Muñoz, M. M., Rodríguez, C. J., Delgado, D. R., Peña, M. Á., Jouyban, A., Martínez, F. (2015). Solubility and saturation apparent specific volume of some sodium sulfonamides in propylene glycol + water mixtures at 298.15 K. Journal of Molecular Liquids, 211, 192–196. https://doi.org/10.1016/j.molliq.2015.07.016
- Pirhayati, F. H., Shayanfar, A., Rahimpour, E., Barzegar-Jalali, M., Martinez, F., Jouyban, A. (2017). Solubility of sildenafil citrate in propylene glycol + water mixtures at various temperatures. Physics and Chemistry of Liquids, 56 (4), 508–517. https://doi.org/10.1080/00319104.2017.1354376
- Miron, D. S., Rădulescu, F. Ștefan, Voicu, V. A., Mînea, A., Cardot, J.-M., Shah, V. P. (2021). Rheological and in vitro release measurements of manufactured acyclovir 5% creams: confirming sensitivity of the in vitro release. Pharmaceutical Development and Technology, 26 (7), 779–787. https://doi.org/10.1080/10837450.2021.1945625
- Benaouda, F., Jones, S. A., Martin, G. P., Brown, M. B. (2015). Localized Epidermal Drug Delivery Induced by Supramolecular Solvent Structuring. Molecular Pharmaceutics, 13 (1), 65–72. https://doi.org/10.1021/acs.molpharmaceut.5b00499
- Bakhbakhi, Y., Charpentier, P., Rohani, S. (2009). The Solubility of Beclomethasone-17,21-dipropionate in Selected Organic Solvents: Experimental Measurement and Thermodynamic Modeling. Organic Process Research & Development, 13 (6), 1322–1326. https://doi.org/10.1021/op900142j
- Derzhavna Farmakopeia Ukrainy. Vol. 2 (2024). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 424.
- ICH Q8 (R2) Pharmaceutical development – Scientific guideline EMEA/CHMP/167068/2004 (2009). European Medicines Agency. Available at: www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-scientific-guideline
- Bezugla, O. P., Lyapunova, A. M., Kirilyuk, I. A., Lyapunov, O. M. (2017). The study of steroid distribution in emulsions by the spin probe method. Clinical pharmacy, 21 (3), 46–54. https://doi.org/10.24959/cphj.17.1430
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Оlena Bezuglaya, Alla Krasnopyorova, Olga Vashchenko, Yurij Stolper, Anna Liapunova, Igor Zinchenko, Oleksii Liapunov, Yuliia Shliapkina, Nikolay Lyapunov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






