Ecological optimization of synthesis routes for a new non covalent inhibitor of SARS CoV 2 main protease as a promising active pharmaceutical ingredient
DOI:
https://doi.org/10.15587/2519-4852.2025.340744Keywords:
SARS-CoV-2 protease inhibitors, optimization of synthesis routes, pharmaceutical substance, environmental parametersAbstract
When transitioning from laboratory synthesis to industrial production, comprehensive research involves more than just assessing quality and biological activity. A crucial aspect of developing a new active pharmaceutical ingredient (API) is the research and optimization of synthetic routes. This process must consider safety, environmental impact, and other parameters set by regulatory requirements.
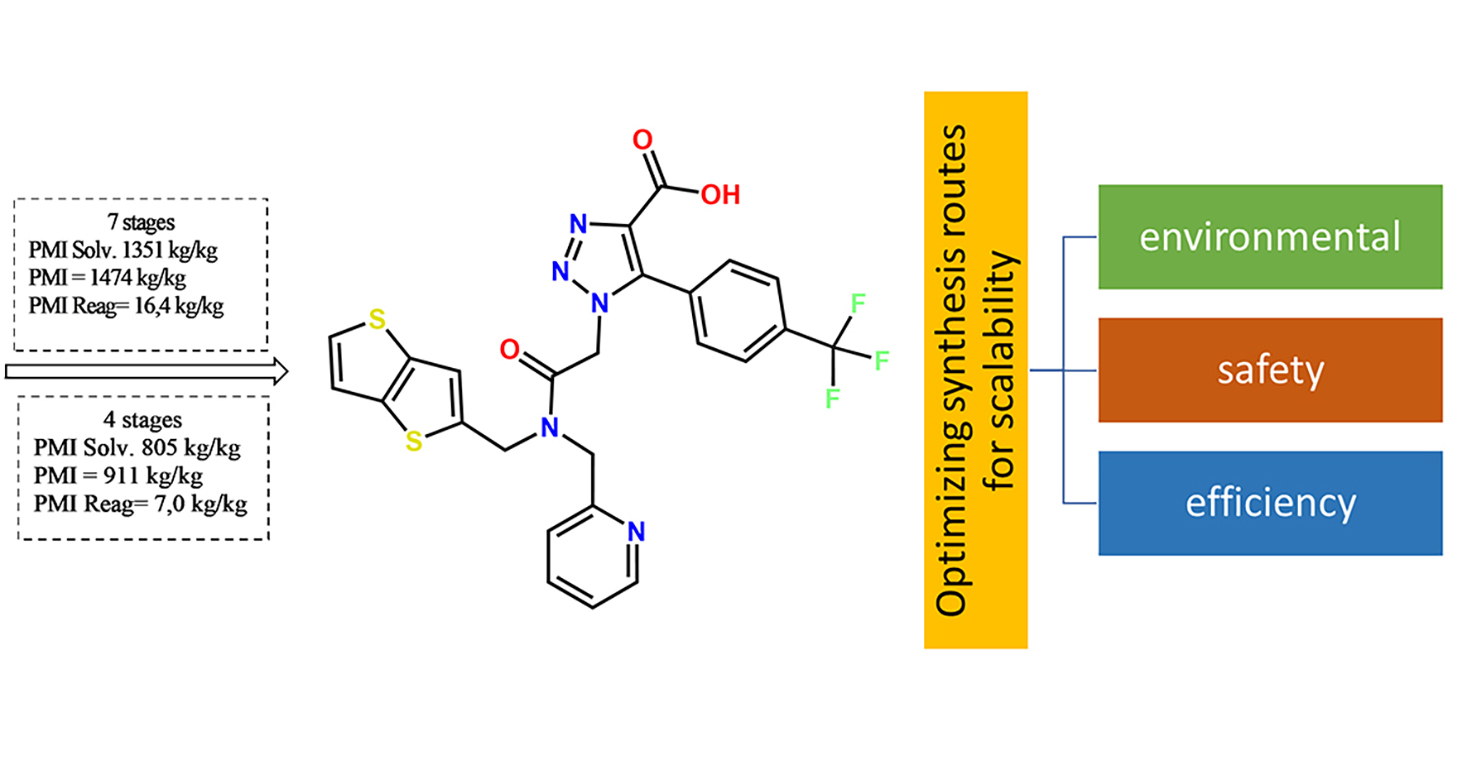
In previous studies, we synthesized a new biologically active substance intended as a non-covalent inhibitor of the main protease of the SARS-CoV-2 virus [1]. In this work, we analyzed and quantitatively assessed the environmental characteristics of the synthetic routes and optimized the synthesis method for further scaling of the technology. To identify the key factors affecting the environmental impact and efficiency of the process, we applied the fundamental principles of “green chemistry”.
The aim of the study is to evaluate and optimize the environmental parameters involved in the synthesis of a new biologically active molecule: 1-(2-oxo-2-((pyridin-2-ylmethyl)(thieno[3,2-b]thiophene-2-ylmethyl)amino)ethyl)-5-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazole-4-carboxylic acid (HIT) [1]. This compound has shown promise as a non-covalent inhibitor of SARS-CoV-2 proteases for potential treatment of COVID-19. The optimization process aims to enhance the synthesis efficiency while also improving the environmental aspects, considering the future scalability of production.
Materials and methods. The study used computational methods, statistical and structural-logical methods, and the EcoScale and DataWarrior software tools.
Results. While studying synthetic routes, alternative solvents were considered, the number of stages was reduced, and the intensity of the process mass was improved.
Conclusions. A comprehensive approach to optimizing synthetic pathways has made it possible to improve the environmental parameters of the target molecule (HIT) synthesis scheme, increase the overall efficiency of the process, and develop safer and more efficient processes for scaling up and producing a new pharmaceutical substance
Supporting Agency
- Grant No. 42/0062 (2021.01/0062) “Molecular design, synthesis and screening of new potential antiviral pharmaceutical ingredients for the treatment of infectious diseases COVID-19” from the National Research Foundation of Ukraine.
References
- Burange, A. S., Osman, S. M., Luque, R. (2022). Understanding flow chemistry for the production of active pharmaceutical ingredients. IScience, 25 (3), 103892. https://doi.org/10.1016/j.isci.2022.103892
- Balaji, R. R. Logic of Organic Synthesis. 3: Criteria for Selection of the Synthetic Route. LibreTextsTM. Chemistry. Available at: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Logic_of_Organic_Synthesis_(Rao)/03%3A_Criteria_for_Selection_of_the_Synthetic_Route
- Harrington, P. J. (2011). Pharmaceutical Process Chemistry for Synthesis: Rethinking the Routes to Scale-Up. John Wiley & Sons, 376. https://doi.org/10.1002/9780470909775
- Butters, M., Catterick, D., Craig, A., Curzons, A., Dale, D., Gillmore, A. et al. (2006). Critical Assessment of Pharmaceutical ProcessesA Rationale for Changing the Synthetic Route. Chemical Reviews, 106 (7), 3002–3027. https://doi.org/10.1021/cr050982w
- Key Considerations for API Process Development and Optimization (2024). Evotec. Available at: https://www.evotec.com/sciencepool/key-considerations-for-api-process-development-and-optimization#asynthetic-route-selection-1
- Taylor, C. J., Pomberger, A., Felton, K. C., Grainger, R., Barecka, M., Chamberlain, T. W. et al. (2023). A Brief Introduction to Chemical Reaction Optimization. Chemical Reviews, 123 (6), 3089–3126. https://doi.org/10.1021/acs.chemrev.2c00798
- ISO 14001:2015. Environmental management systems – Requirements with guidance for use. ISO. Available at: https://www.iso.org/standard/60857.html
- Krueger, J., Dieskau, A. P., Hassfeld, J., Gries, J., Block, O., Weinmann, H. et al. (2025). Chemical Process Development in the Pharmaceutical Industry in Europe – Insights and Perspectives from Industry Scientists. Angewandte Chemie International Edition, 64 (19). https://doi.org/10.1002/anie.202420719
- Prat, D., Wells, A., Hayler, J., Sneddon, H., McElroy, C. R., Abou-Shehada, S., Dunn, P. J. (2016). CHEM21 selection guide of classical- and less classical-solvents. Green Chemistry, 18 (1), 288–296. https://doi.org/10.1039/c5gc01008j
- Globally Harmonized System of Classification and Labelling of Chemicals (2023). GHS Rev. United Nations. Available at: https://unece.org/sites/default/files/2023-07/GHS%20Rev10e.pdf
- Anastas, P. T., Warner, J. C. (1998). Green Chemistry: Theory and Practice. New York: Oxford University Press, 30.
- Gu, Y., Jérôme, F. (2013). Bio-based solvents: an emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chemical Society Reviews, 42 (24), 9550–9570. https://doi.org/10.1039/c3cs60241a
- Armenta, S., Esteve-Turrillas, F. A., Garrigues, S., de la Guardia, M. (2022). Alternative green solvents in sample preparation. Green Analytical Chemistry, 1, 100007. https://doi.org/10.1016/j.greeac.2022.100007
- Kumar, M. K. V., Devi, N., Jothirmae, K., Jyothi, K. L., Kumar, R. T. N. P., Venkatesh, G. (2025) A Review on Green Chemistry: A Sustainable Approach to Chemical Innovation. International Journal of Pharmaceutical Sciences, 3 (3), 350–359. https://doi.org/10.5281/zenodo.14988326
- ST-N MOZU 42-3.0:2011 Likarski zasoby. Farmatsevtychna rozrobka (ICH Q8). Kyiv. Available at: https://compendium.com.ua/uk/clinical-guidelines-uk/standartizatsiya-farmatsevtichnoyi-produktsiyi-tom-1/st-n-mozu-42-3-0-2011/
- Saliy, O., Los, O., Palchevska, T., Nebylytsia, K. (2021). Implementation of the Quality by Design approach for developing the composition and the manufacturing technology of an injectable drug for intra-articular introduction. News of Pharmacy, 1 (101), 28–37. https://doi.org/10.24959/nphj.21.44
- Derzhavna Farmakopeia Ukrainy. Vol. 1. Kharkiv: Derzhavne pidpryiemstvo «Ukrayinskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- ICH Q3C (R9) Guideline on impurities guideline for residual solvents – Step 5 (2024). Reference Number: EMA/CHMP/ICH/82260/2006. Available at: https://www.ema.europa.eu/en/ich-q3c-r9-residual-solvents-scientific-guideline
- Pleissner, D., Kümmerer, K. (2018). Green Chemistry and Its Contribution to Industrial Biotechnology. Sustainability and Life Cycle Assessment in Industrial Biotechnology. Cham: Springer, 281–298. https://doi.org/10.1007/10_2018_73
- Van Aken, K., Strekowski, L., Patiny, L. (2006). EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein Journal of Organic Chemistry, 2. https://doi.org/10.1186/1860-5397-2-3
- Process Mass Intensity (PMI). Available at: https://acsgcipr.org/tools/process-mass-intensity
- Kyrychenko, A. V., Geleverya, A. O., Yevsieiva, L. V., Kovalenko, S. M., Kalugin, O. N. (2024). Anti-COVID-19 drug discovery and de novo molecular generation of evolutionary libraries of non-covalent inhibitors of main protease of coronavirus SARS-COV-2. Modern pharmacy: current realities and prospects for development. Odessa: Odessa National University named after I. I. Mechnikov: Book of Abstracts, 79–80.
- The EcoScale. ecoscale.cheminfo.org. Available at: https://ecoscale.cheminfo.org/man
- Thieno3,2-b.thiophene-2-carboxaldehyde. Chemical Book. Available at: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB7506778.htm
- -oxo-3-(4-trifluoromethylphenyl)propionic acid ethyl ester. Chemical Book. Available at: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB6763392.htm
- The CHEM21 Solvent Selection Guide. Available at: https://learning.acsgcipr.org/guides-and-metrics/solvent-selection-guides/the-chem21-solvent-selection-guide
- Monrealskii protokol po veshchestvam, razrushaiushchim ozonovyi sloi. OON. Available at: https://www.un.org/ru/documents/decl_conv/conventions/montreal_prot.shtml
- Environmentally friendly fluorinated specialty chemicals: Electronic fluorinated fluids (2023). Fluorined-chemicals. Available at: https://ua.fluorines-chemicals.com/info/environmentally-friendly-fluorinated-specialty-86026247.html
- Haynes, W. M. (Ed.) (2014). CRC Handbook of Chemistry and Physics. CRC Press, 2704. https://doi.org/10.1201/b17118
- CRC Handbook of Chemistry and Physics, 92nd Edition. (2011). CRC Press, 2656. https://doi.org/10.1201/b17379
- tert-Butyl methyl ether. Chemical Book. https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2853178.htm
- Yevsieieva, L. V., Lohachova, K. O., Kyrychenko, A., Kovalenko, S. M., Ivanov, V. V., Kalugin, O. N. (2023). Main and papain-like proteases as prospective targets for pharmacological treatment of coronavirus SARS-CoV-2. RSC Advances, 13 (50), 35500–35524. https://doi.org/10.1039/d3ra06479d
- Yevsieieva, L., Trostianko, P., Kyrychenko, A., Ivanov, V., Kovalenko, S., Kalugin, O. (2024). Design of non-covalent dual-acting inhibitors for proteases MPRO and PLPRO of coronavirus SARS-CoV-2 through evolutionary library generation, pharmacophore profile matching, and molecular docking calculations. ScienceRise: Pharmaceutical Science, 6 (52), 15–26. https://doi.org/10.15587/2519-4852.2024.313808
- Lohachova, K. O., Sviatenko, A. S., Kyrychenko, A., Ivanov, V. V., Langer, T., Kovalenko, S. M., Kalugin, O. N. (2024). Computer-aided drug design of novel nirmatrelvir analogs inhibiting main protease of Coronavirus SARS-CoV-2. Journal of Applied Pharmaceutical Science, 14 (5), 232–239. https://doi.org/10.7324/japs.2024.158114
- Yevsieieva, L., Kyrychenko, A., Trostianko, P., Ivanov, V., Kovalenko, S. M., Kalugin, O. (2025). Targeted structural design of molecular scaffolds for dual-action peptidomimetic inhibitors against SARS-CoV-2 MPRO and PLPRO. ScienceRise: Pharmaceutical Science, 5 (57), 56–67. https://doi.org/10.15587/2519-4852.2025.337951
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Larysa Yevsieieva, Anna Geleverya, Nataliia Koval, Alexander Kyrychenko, Sergiy M. Kovalenko, Oleg N. Kalugin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






