Antibacterial and antibiofilm activity of Albizia lebbeck leaves extracts in Pseudomonas aeruginosa isolated from urinary tract infection
DOI:
https://doi.org/10.15587/2519-4852.2025.342374Keywords:
Albizia lebbeck, Antibiofilm activity, Antibacterial activity, Antioxidant activity, Total phenolic content, Pseudomonas aeruginosaAbstract
Microbial infections have become one of the most pressing public health issues worldwide as a result of the emergence of resistance to current antibiotics. This has prompted scientists to examine the antibacterial characteristics of medicinal plants.
The aim of the study: Extract secondary metabolites from Albizia lebbeck and analyze how they affect Pseudomonas aeruginosa.
Materials and methods: From the labs of the University of Baghdad's Genetic Eng. and Biotech. Institute, 10 isolates of P. aeruginosa were obtained. The identification was confirmed by cultivating the isolates on cetrimide agar and using the VITEK2 technology.
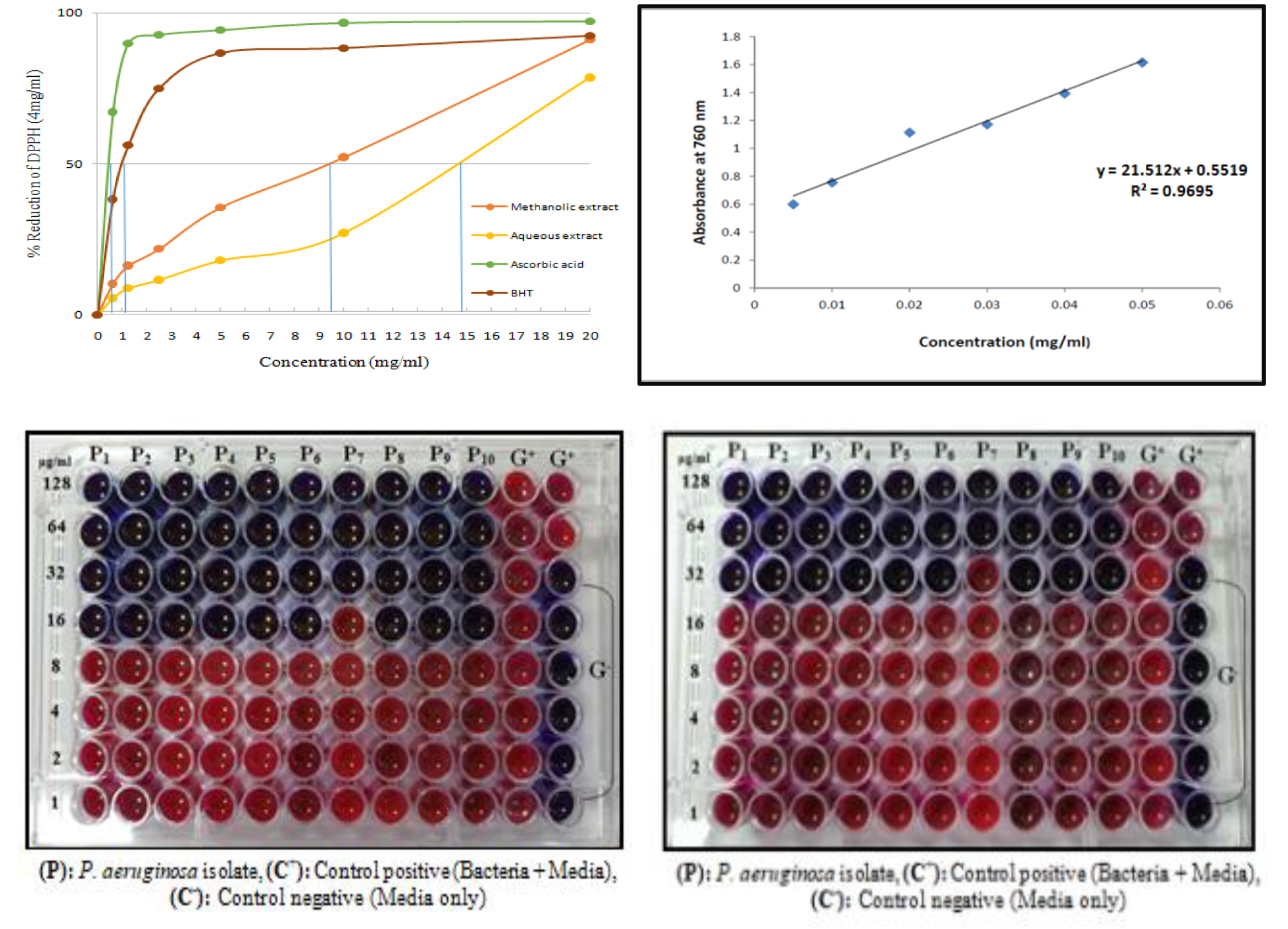
Results: The chemical analysis of the methanolic and aqueous extracts revealed the presence of secondary metabolite molecules such as alkaloids, flavonoids, glycosides, phenols, saponins, and tannins. The total phenols content of the methanolic and aqueous extracts was 71.11 mg/g and 45.15 mg/g, respectively. Furthermore, the results indicated that at a concentration of 50 mg/ml, the methanolic extract outperformed the aqueous extract in free radical scavenging by 78. 65% to 91. 20%. Using the disk diffusion method, the methanolic extract also demonstrated higher antibacterial activity than the aqueous extract of Albizia lebbeck leaves, and its potency increased with increasing concentration. The methanolic extract's lowest inhibitory concentration against P. aeruginosa isolates was determined to be 16 mg/ml, whereas the aqueous extract's was 32 mg/ml. According to the biofilm formation experiment, the methanol extract inhibits the formation of biofilms at a concentration of 200 mg/ml, whereas the aqueous extract does so at a concentration of 400 mg/ml.
Conclusion: This work demonstrated that the secondary metabolites extracted from Albizia lebbeck leaves have a considerable antibacterial and antibiofilm action on P. aeruginosa, even though the bacterial isolates create a strong biofilm. This research has shown that Albizia lebbeck leaves can be used in traditional therapy for bacterial infections and diseases caused by oxidative stress since they contain therapeutic phytochemicals with strong antibacterial and antioxidant effects
References
- Jardak, M., Elloumi-Mseddi, J., Aifa, S., Mnif, S. (2017). Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids in Health and Disease, 16 (1). https://doi.org/10.1186/s12944-017-0580-9
- Jafari-Sales, A., Shahniani, A., Fathi, R., Malekzadeh, P., Mobaiyen, H., Rasi Bonab, F. (2017). Evaluation of Antibacterial Activity of Essential Oil of Ziziphora clinopodioides and Achillea wilhelmsii on Antibiotic-resistant Strains of Staphylococcus aureus. Internal Medicine and Medical Investigation Journal, 2 (2), 49–56. https://doi.org/10.24200/imminv.v2i2.58
- Mobaiyen, H., Jafari Sales, A., Sayyahi, J. (2016). Evaluating antimicrobial effects of centaurea plant’s essential oil on pathogenic bacteria: staphylococcus aureus, staphylococcus epidermidis, and escherichia coli isolated from clinical specimens. Journal of Fasa University of Medical Sciences, 5 (4), 479–487. Available at: https://dor.isc.ac/dor/20.1001.1.22285105.2016.5.4.1.6.
- AL-Azawi, A. H. (2017). Phytochemical, Antibacterial and antioxidant activities of dodonea viscosa Jacq. extracts cultivated in Iraq. Iraqi Journal of Biotechnology, 16 (4), 37–46. Available at: https://jige.uobaghdad.edu.iq/index.php/IJB/article/view/154
- Yasin, S. A., AL-Azawi, A. H. (2019). Antibacterial activity of Conocarpus Erectus leaves extracts on some microorganisms isolated from patients with burn infection. Plant Archives, 19 (2), 583–589. Available at: https://repository.uobaghdad.edu.iq/file/publication/draft/ee9929ca-ea94-4b2e-b3ca-5d83635d687b.pdf
- Mishra, S. S., Gothecha, V. K., Sharma, A. (2010). Albizia lebbeck: a short review. Journal of herbal medicine and toxicology, 4 (2), 9–15. Available at: https://www.doc-developpement-durable.org/file/Culture/Arbres-Bois-de-Rapport-Reforestation/FICHES_ARBRES/albizia%20lebbeck/Albizia%20lebbeck%20a%20short%20review.pdf
- Basic laboratory procedures in clinical bacteriology (2003). World Health Organization.
- Performance Standards for Antimicrobial Susceptibility Testing; CLSI Supplement, CLSI M100-28thed. (2019). Clinical and Laboratory Standareds Institute Wayne, 39 (1).
- Patel, F., Goswami, P., Khara, R. (2016). Detection of Biofilm formation in device associated clinical bacterial isolates in cancer patients. Sri Lankan Journal of Infectious Diseases, 6 (1), 43–50. https://doi.org/10.4038/sljid.v6i1.8086
- Kırmusaoğlu, S. (2019). The Methods for Detection of Biofilm and Screening Antibiofilm Activity of Agents. Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods. https://doi.org/10.5772/intechopen.84411
- N’Guessan, J., Bidié, A., Lenta, B., Weniger, B., André, P., Guédé-Guina, F. (2010). In vitro assays for bioactivity-guided isolation of anti-salmonella and antioxidant compounds in Thon ninja sanguine flowers. African Journal of Biotechnology, 6, 1685–1689. Available at: https://www.ajol.info/index.php/ajb/article/view/57753/0
- Method 08-01. The Association St. Paul, M. N (1984). American Association of Cereal Chemists (AACC).
- Suleiman, M. H. A., Ateeg, A. A. (2020). Antimicrobial and Antioxidant Activities of Different Extracts from Different Parts of Zilla spinosa (L.) Prantl. Evidence-Based Complementary and Alternative Medicine, 2020 (1). https://doi.org/10.1155/2020/6690433
- Jayaprakasha, G. K., Singh, R. P., Sakariah, K. K. (2001). Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chemistry, 73 (3), 285–290. https://doi.org/10.1016/s0308-8146(00)00298-3
- Al-Azawi, A. H., Kais, K. G., Salih, H. H. (2018). Phytochemical, antibacterial and antioxidant activities of Capparis spinosa L. Cultivated in Iraq. Bioscience Research, 15 (3), 2611–2618. Available at: https://repository.uobaghdad.edu.iq/articles/yxeIv5EBVTCNdQwCXpkn?page=3
- Ogunmoyole, T., Inaboya, S., Makun, J. O., Kade, I. J. (2013). Differential antioxidant properties of ethanol and water soluble phytochemicals of false nutmeg (Monodora myristica) seeds. International Journal of Biotechnology and Biochemistry, 2, 253–262. Available at: https://www.internationalscholarsjournals.com/articles/differential-antioxidant-properties-of-ethanol-and-water-soluble-phytochemicals-of-false-nutmeg-monodora-myristica-seeds.pdf
- Razmavar, S., Abdulla, M. A., Ismail, S. B., Hassandarvish, P. (2014). Antibacterial Activity of Leaf Extracts ofBaeckea frutescensagainst Methicillin-ResistantStaphylococcus aureus. BioMed Research International, 2014, 1–5. https://doi.org/10.1155/2014/521287
- Ohikhena, F. U., Wintola, O. A., Afolayan, A. J. (2017). Evaluation of the Antibacterial and Antifungal Properties of Phragmanthera capitata (Sprengel) Balle (Loranthaceae), a Mistletoe Growing on Rubber Tree, Using the Dilution Techniques. The Scientific World Journal, 2017, 1–8. https://doi.org/10.1155/2017/9658598
- Saderi, H., Owlia, P. (2015). Detection of multidrug resistant (MDR) and extremely drug resistant (XDR) P. aeruginosa isolated from patients in Tehran, Iran. Iranian journal of pathology, 10 (4), 265–271. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4539747/
- Mousa, K. A., AL-Azawi, A. H. (2025). Evaluation Antibacterial Activity of Quercetin Against XDR – Pseudomonas aeruginosa. Iraqi Journal of Biotechnology, 24 (1), 103–112. Available at: https://jige.uobaghdad.edu.iq/index.php/IJB/article/view/800
- de Almeida Silva, K. de C. F., Calomino, M. A., Deutsch, G., de Castilho, S. R., de Paula, G. R., Esper, L. M. R., Teixeira, L. A. (2017). Molecular characterization of multidrug-resistant (MDR) Pseudomonas aeruginosa isolated in a burn center. Burns, 43 (1), 137–143. https://doi.org/10.1016/j.burns.2016.07.002
- Yekani, M., Memar, M. Y., Alizadeh, N., Safaei, N., Ghotaslou, R. (2017). Antibiotic resistance patterns of biofilm-forming Pseudomonas aeruginosa isolates from mechanically ventilated patients. International Journal of Scientific Study, 5 (5), 84–88.
- Rasamiravaka, T., Labtani, Q., Duez, P., El Jaziri, M. (2015). The Formation of Biofilms byPseudomonas aeruginosa: A Review of the Natural and Synthetic Compounds Interfering with Control Mechanisms. BioMed Research International, 2015, 1–17. https://doi.org/10.1155/2015/759348
- Asadzadegan, R., Haratian, N., Sadeghi, M., Maroufizadeh, S., Mobayen, M., Sedigh Ebrahim Saraei, H., Hasannejad‐Bibalan, M. (2023). RETRACTED: Antibiofilm and antimicrobial activity of Lactobacillus cell free supernatant against Pseudomonas aeruginosa isolated from burn wounds. International Wound Journal, 20 (10), 4112–4121. https://doi.org/10.1111/iwj.14305
- Abd Aziz, N. A., Hasham, R., Sarmidi, M. R., Suhaimi, S. H., Idris, M. K. H. (2021). A review on extraction techniques and therapeutic value of polar bioactives from Asian medicinal herbs: Case study on Orthosiphon aristatus, Eurycoma longifolia and Andrographis paniculata. Saudi Pharmaceutical Journal, 29 (2), 143–165. https://doi.org/10.1016/j.jsps.2020.12.016
- Nawaz, H., Shad, M. A., Rehman, N., Andaleeb, H., Ullah, N. (2020). Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Brazilian Journal of Pharmaceutical Sciences, 56. https://doi.org/10.1590/s2175-97902019000417129
- Obakiro, S. B., Kiprop, A., Kigondu, E., K’Owino, I., Odero, M. P., Manyim, S. et al. (2021). Traditional Medicinal Uses, Phytoconstituents, Bioactivities, and Toxicities of Erythrina abyssinica Lam. ex DC. (Fabaceae): A Systematic Review. Evidence-Based Complementary and Alternative Medicine, 2021, 1–43. https://doi.org/10.1155/2021/5513484
- Kallscheuer, N., Vogt, M., Marienhagen, J. (2017). A Novel Synthetic Pathway Enables Microbial Production of Polyphenols Independent from the Endogenous Aromatic Amino Acid Metabolism. ACS Synthetic Biology, 6 (3), 410–415. https://doi.org/10.1021/acssynbio.6b00291
- Fitriana, W. D., Ersam, T., Shimizu, K., Fatmawati, S. (2018). Antioxidant Activity of Moringa oleifera Extracts. Indonesian Journal of Chemistry, 16 (3), 297–301. https://doi.org/10.22146/ijc.21145
- Raza, A. M., Anwar, F., Shahwar, D., Mumtaz, W. M., Danish, M., Nazar, F. M. (2016). Antioxidant and antiacetylcholine esterase potential of aerial parts of Conocarpus erectus, Ficus variegata and Ficus maclellandii. Pakistan Journal of Pharmaceutical Sciences, 29 (2), 489–495.
- Shanaida, M., Golembiovska, O., Hudz, N., Wieczorek, P. P. (2018). Phenolic compounds of herbal infusions obtained from some species of theLamiaceaefamily. Current Issues in Pharmacy and Medical Sciences, 31 (4), 194–199. https://doi.org/10.1515/cipms-2018-0036
- Al-Halbosiy, M. M. F., Hasan, Z. Y. M., Mohammad, F. I., Abdulhameed, B. A. (2020). Biological Activities of Iraqi Fig (Ficuscarica) CrudeEthanolic and Total Flavonoids Extracts. Iraqi Journal of Science, 1612–1621. https://doi.org/10.24996/ijs.2020.61.7.9
- Flieger, J., Flieger, M. (2020). The [DPPH●/DPPH-H]-HPLC-DAD Method on Tracking the Antioxidant Activity of Pure Antioxidants and Goutweed (Aegopodium podagraria L.) Hydroalcoholic Extracts. Molecules, 25 (24), 6005. https://doi.org/10.3390/molecules25246005
- Falana, M. B., Nurudeen, Q. O. (2022). Phytochemical Screening and in Vitro Antimicrobial Activities of Euphorbia Lateriflora on Selected Pathogens. Iraqi Journal of Science, 1402–1412. https://doi.org/10.24996/ijs.2022.63.4.1
- Adeeyo, A. O., Edokpayi, J. N., Alabi, M. A., Msagati, T. A. M., Odiyo, J. O. (2021). Plant active products and emerging interventions in water potabilisation: disinfection and multi-drug resistant pathogen treatment. Clinical Phytoscience, 7 (1). https://doi.org/10.1186/s40816-021-00258-4
- Daglia, M. (2012). Polyphenols as antimicrobial agents. Current Opinion in Biotechnology, 23 (2), 174–181. https://doi.org/10.1016/j.copbio.2011.08.007
- Yi, Z.-B., Yan Yu, Liang, Y.-Z., Bao Zeng. (2007). Evaluation of the antimicrobial mode of berberine by LC/ESI-MS combined with principal component analysis. Journal of Pharmaceutical and Biomedical Analysis, 44 (1), 301–304. https://doi.org/10.1016/j.jpba.2007.02.018
- Panda, P., Tripathy, G. (2009). Wound healing activity of aqueous and methanolic bark extract of Vernonia arborea in Wistar rats. Natural Products Radiance, 8, 6–11. Available at: https://api.semanticscholar.org/CorpusID:55470519
- Engels, C., Schieber, A., Gänzle, M. G. (2011). Inhibitory Spectra and Modes of Antimicrobial Action of Gallotannins from Mango Kernels (Mangifera indicaL.). Applied and Environmental Microbiology, 77 (7), 2215–2223. https://doi.org/10.1128/aem.02521-10
- Ncube, N. S., Afolayan, A. J., Okoh, A. I. (2008). Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African Journal of Biotechnology, 7 (12), 1797–1806. https://doi.org/10.5897/ajb07.613
- Mohamed, I. E., AL-Azawi, A. H. (2022). Evaluation of Antibacterial Activity of Laurus nobilis Leaves Extract against Escherichia coli Isolates. Iraqi Journal of Biotechnology, 21 (2), 623–631. Available at: https://jige.uobaghdad.edu.iq/index.php/IJB/article/view/538
- Górniak, I., Bartoszewski, R., Króliczewski, J. (2018). Comprehensive review of antimicrobial activities of plant flavonoids. Phytochemistry Reviews, 18 (1), 241–272. https://doi.org/10.1007/s11101-018-9591-z
- Mahdi, L. F., AL-Azawi, A. H. (2022). Antibiofilm Activity of Conocarpus erectus Leaves Extract and Assessment Its Effect on pelA and algD Genes on Multi-drug Resistant Pseudomonas aeruginosa. The Egyptian Journal of Hospital Medicine, 89 (2), 6961–6969. https://doi.org/10.21608/ejhm.2022.271919
- Al-Deen, F. M. (2017). Evolution of antibacterial activity of various Solvents Extracts of Annona squamosa fruit. Iraqi Journal of Science, 58 (4C), 2301–2308. Available at: https://ijs.uobaghdad.edu.iq/index.php/eijs/article/view/62.
- Alam, K., Farraj, D. A. A., Mah-e-Fatima, S., Yameen, M. A., Elshikh, M. S., Alkufeidy, R. M. et al. (2020). Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. Journal of Infection and Public Health, 13 (11), 1734–1741. https://doi.org/10.1016/j.jiph.2020.07.007
- Al-Aboudi, Z. F., AL-Azawi, A. H. (2025). Antibacterial and Antibiofilm Activity of Phenolic Compounds Extracted from Camellia sinensis and Evaluate its Effect on the Gene Expression of pelA Gene in Pseudomonas aeruginosa. Iraqi Journal of Biotechnology, 24 (2), 55–70. Available at: https://jige.uobaghdad.edu.iq/index.php/IJB/article/view/842
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Inas Humam Abdulaal, Ahmed H. AL-Azawi, Diana Ghanem Yahya

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






