Acetylcholinesterase-targeted biogenic–thienopyrimidine hybrids: design, synthesis and pharmacological evaluation of compounds with anti-amnestic, anxiolytic-like and pain-modulating properties
DOI:
https://doi.org/10.15587/2519-4852.2025.346823Keywords:
Thienopyrimidine, neurodegeneration, acetylcholinesterase, glycine, amnesiaAbstract
The aim. To carry out the rational design, synthesis, and experimental evaluation of new glycine-linked thieno[2,3-d]pyrimidine hybrids as potential modulators of memory, anxiety, and pain for further correction of neurodegenerative processes, integrating in silico and in vivo investigations.
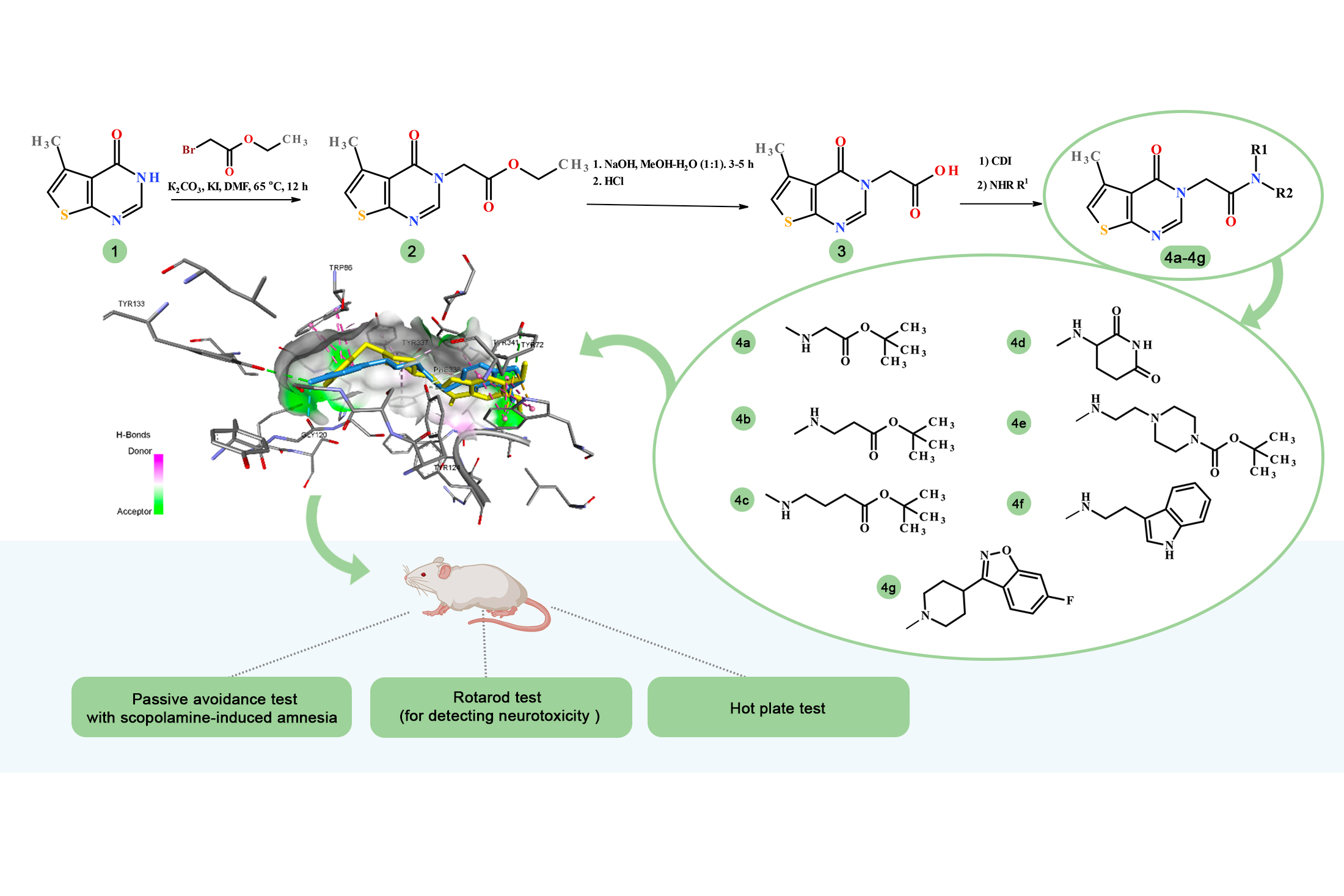
Materials and Methods. Organic synthesis methods; structure confirmation by ¹H, ¹³C NMR spectroscopy, LC-MS, and elemental analysis. Molecular docking was performed using AutoDock Vina, AutoDockTools 1.5.6, and Discovery Studio Client. The pharmacological studies were carried out using a scopolamine-induced amnesia model and included the following behavioral assays: the Passive Avoidance Test, the Light-dark Transition Test, the Rotarod Test, and the Hot Plate Test.
Results and Discussion. A series of newly designed glycine-linked thieno[2,3-d]pyrimidine hybrids was rationally developed, synthesized, and evaluated as potential modulators of neurodegenerative processes. The synthetic procedures for obtaining the intermediates and target 5-methylthieno[2,3-d]pyrimidin-4(3H)-one hybrids via amide coupling were optimized. Molecular docking to AChE (PDB ID: 7E3H) revealed that several derivatives, particularly 4d, 4f, and 4g, exhibited favorable binding energies (down to –12.5 kcal/mol) and formed an extensive network of hydrogen-bonding, halogen, π–π, and hydrophobic interactions within the active site of acetylcholinesterase. In vivo studies using the scopolamine-induced amnesia model demonstrated that these compounds display moderate anti-amnestic (pro-cognitive) properties and do not influence motor coordination or nociceptive sensitivity. Compound 4g showed anti-amnestic activity comparable to that of Donepezil, as well as pronounced anxiolytic effects. A correlation between the in silico predictions and the in vivo findings was established.
Conclusions. The rational design, synthesis, and structural characterization of new AChE-targeted inhibitors, combined with in silico calculations and subsequent in vivo validation, enabled the identification of several thieno[2,3-d]pyrimidine derivatives with moderate anti-amnestic properties in the scopolamine-induced amnesia model, highlighting their potential as promising structures for further optimization
Supporting Agency
- The research was funded by the Ministry of Health of Ukraine at the expense of the State Budget in the framework # 2301020 “Scientific and scientific-technical activity in the field of health protection” on the topic «Molecular modelling and synthesis of innovative pyrimidine derivatives as promising agents for the treatment of neurodegenerative diseases» (State registration number: 0124U002006. Order of the Ministry of Health of Ukraine of January 16, 2024, No. 82)
References
- Arlt, S. (2013). Non-Alzheimer’s disease – related memory impairment and dementia. Dialogues in Clinical Neuroscience, 15 (4), 465–473. https://doi.org/10.31887/dcns.2013.15.4/sarlt
- Morais, R., Pires, R., Jesus, T., Lemos, R., Duro, D., Lima, M. et al. (2024). Cognitive Impairment in Neurodegenerative Diseases: A Trans-Diagnostic Approach Using a Lesion-Symptom Mapping Analysis. Neuroscience. https://doi.org/10.2139/ssrn.5030776
- Pentkowski, N. S., Rogge-Obando, K. K., Donaldson, T. N., Bouquin, S. J., Clark, B. J. (2021). Anxiety and Alzheimer’s disease: Behavioral analysis and neural basis in rodent models of Alzheimer’s-related neuropathology. Neuroscience & Biobehavioral Reviews, 127, 647–658. https://doi.org/10.1016/j.neubiorev.2021.05.005
- Blanton, H., Reddy, P. H., Benamar, K. (2023). Chronic pain in Alzheimer’s disease: Endocannabinoid system. Experimental Neurology, 360, 114287. https://doi.org/10.1016/j.expneurol.2022.114287
- Aillaud, I., Kaniyappan, S., Chandupatla, R. R., Ramirez, L. M., Alkhashrom, S., Eichler, J. et al. (2022). A novel D-amino acid peptide with therapeutic potential (ISAD1) inhibits aggregation of neurotoxic disease-relevant mutant Tau and prevents Tau toxicity in vitro. Alzheimer’s Research & Therapy, 14 (1). https://doi.org/10.1186/s13195-022-00959-z
- Ullah, R., Jo, M. H., Riaz, M., Alam, S. I., Saeed, K., Ali, W. et al. (2020). Glycine, the smallest amino acid, confers neuroprotection against d-galactose-induced neurodegeneration and memory impairment by regulating c-Jun N-terminal kinase in the mouse brain. Journal of Neuroinflammation, 17 (1). https://doi.org/10.1186/s12974-020-01989-w
- Salek, R. M., Xia, J., Innes, A., Sweatman, B. C., Adalbert, R., Randle, S. et al. (2010). A metabolomic study of the CRND8 transgenic mouse model of Alzheimer’s disease. Neurochemistry International, 56 (8), 937–947. https://doi.org/10.1016/j.neuint.2010.04.001
- Li, H., Ye, D., Xie, W., Hua, F., Yang, Y., Wu, J. et al. (2018). Defect of branched-chain amino acid metabolism promotes the development of Alzheimer’s disease by targeting the mTOR signaling. Bioscience Reports, 38 (4). https://doi.org/10.1042/bsr20180127
- Kazim, S. F., Iqbal, K. (2016). Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: emerging therapeutic modality for Alzheimer’s disease. Molecular Neurodegeneration, 11 (1). https://doi.org/10.1186/s13024-016-0119-y
- Akazawa, N., Hamasaki, A., Tanahashi, K., Kosaki, K., Yoshikawa, T., Myoenzono, K., Maeda, S. (2018). Lactotripeptide ingestion increases cerebral blood flow velocity in middle-aged and older adults. Nutrition Research, 53, 61–66. https://doi.org/10.1016/j.nutres.2018.03.009
- Min, L.-J., Kobayashi, Y., Mogi, M., Tsukuda, K., Yamada, A., Yamauchi, K. et al. (2017). Administration of bovine casein-derived peptide prevents cognitive decline in Alzheimer disease model mice. PLOS ONE, 12 (2), e0171515. https://doi.org/10.1371/journal.pone.0171515
- Singh, Y. P., Kumar, H. (2023). Tryptamine: A privileged scaffold for the management of Alzheimer’s disease. Drug Development Research, 84 (8), 1578–1594. Portico. https://doi.org/10.1002/ddr.22111
- Wu, Y.-L., Yoshida, M., Emoto, H., Ishii, H., Koga, K., Tanaka, M. (2000). Effects of Acute and Chronic Administration of MCI-225, a New Selective Noradrenaline Reuptake Inhibitor With 5-HT3 Receptor Blocking Action, on Extracellular Noradrenaline Levels in the Hypothalamus of Stressed Rats. Japanese Journal of Pharmacology, 83 (1), 31–38. https://doi.org/10.1016/s0021-5198(19)30624-9
- Galvani, F., Cammarota, M., Vacondio, F., Rivara, S., Boscia, F. (2024). Protective Activity of Melatonin Combinations and Melatonin‐Based Hybrid Molecules in Neurodegenerative Diseases. Journal of Pineal Research, 76 (8). https://doi.org/10.1111/jpi.70008
- Babazadeh, A., Vahed, F. M., Liu, Q., Siddiqui, S. A., Kharazmi, M. S., Jafari, S. M. (2023). Natural Bioactive Molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases. ACS Omega, 8 (4), 3667–3683. https://doi.org/10.1021/acsomega.2c06098
- Singh, A. A., Khan, F., Song, M. (2025). Alleviation of Neurological Disorders by Targeting Neurodegenerative-Associated Enzymes: Natural and Synthetic Molecules. International Journal of Molecular Sciences, 26 (10), 4707. https://doi.org/10.3390/ijms26104707
- Ortiz, C. J. C., Damasio, C. M., Pruccoli, L., Nadur, N. F., de Azevedo, L. L., Guedes, I. A. et al. (2020). Cinnamoyl-N-Acylhydrazone-Donepezil Hybrids: Synthesis and Evaluation of Novel Multifunctional Ligands Against Neurodegenerative Diseases. Neurochemical Research, 45 (12), 3003–3020. https://doi.org/10.1007/s11064-020-03148-2
- Fotopoulos, I., Pontiki, E., Hadjipavlou-Litina, D. (2024). Pharmacochemical Study of Multitarget Amino Acids’ Hybrids: Design, Synthesis, In vitro, and In silico Studies. Medicinal Chemistry, 20 (7), 709–720. https://doi.org/10.2174/0115734064279653240125081042
- Fontana, I. C., Souza, D. G., Souza, D. O., Gee, A., Zimmer, E. R., Bongarzone, S. (2023). A Medicinal Chemistry Perspective on Excitatory Amino Acid Transporter 2 Dysfunction in Neurodegenerative Diseases. Journal of Medicinal Chemistry, 66 (4), 2330–2346. https://doi.org/10.1021/acs.jmedchem.2c01572
- Paul, S. S., Biswas, G. (2021). A Mini-Review on the Effectiveness of Peptoids as Therapeutic Interventions against Neurodegenerative Diseases. Current Protein & Peptide Science, 22 (7), 526–533. https://doi.org/10.2174/1389203722666210615125852
- Hatvate, N. T., Shevkar, T. S., Akolkar, H.; Kulkarni, S., Akolkar, H., Khedkar, V., Darekar, N. (Eds.) (2024). Thienopyrimidines Exploring the Chemistry and Bioactivity. Examining Biological Relevance of Fused S-Heterocycles. IGI Global Scientific Publishing, 221–272. https://doi.org/10.4018/979-8-3693-7520-4.ch008
- Kassab, A. E., Gedawy, E. M., Sayed, A. S. (2024). Fused thiophene as a privileged scaffold: A review on anti-Alzheimer’s disease potentials via targeting cholinesterases, monoamine oxidases, glycogen synthase kinase-3, and Aβ aggregation. International Journal of Biological Macromolecules, 265, 131018. https://doi.org/10.1016/j.ijbiomac.2024.131018
- Eissa, K. I., Kamel, M. M., Mohamed, L. W., Doghish, A. S., Alnajjar, R., Al‐Karmalawy, A. A., Kassab, A. E. (2023). Design, synthesis, and biological evaluation of thienopyrimidine derivatives as multifunctional agents against Alzheimer’s disease. Drug Development Research, 84 (5), 937–961. https://doi.org/10.1002/ddr.22064
- DDP-225. DrugBank. Available at: https://go.drugbank.com/drugs/DB05642 Last accessed: 12.06.2025
- Banks, W. A., Rhea, E. M., Reed, M. J., Erickson, M. A. (2024). The penetration of therapeutics across the blood-brain barrier: Classic case studies and clinical implications. Cell Reports Medicine, 5 (11), 101760. https://doi.org/10.1016/j.xcrm.2024.101760
- Ahunovych, V., Klipkov, A. A., Bugera, M., Tarasenko, K., Trofymchuk, S., Razhyk, B. et al. (2024). CF3-Cyclobutanes: Synthesis, Properties, and Evaluation as a Unique tert-Butyl Group Analogue. JACS Au, 4 (11), 4507–4517. https://doi.org/10.1021/jacsau.4c00864
- Novotná, K., Tenora, L., Prchalová, E., Paule, J., Alt, J., Veeravalli, V. et al. (2023). Discovery of tert-Butyl Ester Based 6-Diazo-5-oxo-l-norleucine Prodrugs for Enhanced Metabolic Stability and Tumor Delivery. Journal of Medicinal Chemistry, 66 (22), 15493–15510. https://doi.org/10.1021/acs.jmedchem.3c01681
- Salga, S. M., Ali, H. M., Abdullah, M. A., Abdelwahab, S. I., Wai, L. K., Buckle, M. J. C. et al. (2011). Synthesis, Characterization, Acetylcholinesterase Inhibition, Molecular Modeling and Antioxidant Activities of Some Novel Schiff Bases Derived from 1-(2-Ketoiminoethyl)piperazines. Molecules, 16 (11), 9316–9330. https://doi.org/10.3390/molecules16119316
- -Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride. Ossila. Available at: https://www.ossila.com/products/6-fluoro-3-4-piperidinyl-1-2-benzisoxazole-hydrochloride Last accessed: 17.06.2025
- Cen, L., Wu, Y., He, M., Huang, J., Ren, W., Liu, B. et al. (2025). Discovery and Optimization of Novel Apo-IDO1 Inhibitors by a Pharmacophore-Based Structural Simplification Strategy. Journal of Medicinal Chemistry, 68 (6), 6633–6655. https://doi.org/10.1021/acs.jmedchem.5c00034
- Shaker, M., Elhamifar, D. (2020). Pd-containing IL-based ordered nanostructured organosilica: A powerful and recoverable catalyst for Sonogashira reaction. Tetrahedron Letters, 61 (47), 152481. https://doi.org/10.1016/j.tetlet.2020.152481
- Bassetto, M., Leyssen, P., Neyts, J., Yerukhimovich, M. M., Frick, D. N., Brancale, A. (2016). Computer-aided identification, synthesis and evaluation of substituted thienopyrimidines as novel inhibitors of HCV replication. European Journal of Medicinal Chemistry, 123, 31–47. https://doi.org/10.1016/j.ejmech.2016.07.035
- Lou, J., Liu, Z., Li, Y., Zhou, M., Zhang, Z., Zheng, S. et al. (2011). Synthesis and anti-tumor activities of N′-benzylidene-2-(4-oxothieno[2,3-d]pyrimidin-3(4H)-yl)acetohydrazone derivatives. Bioorganic & Medicinal Chemistry Letters, 21 (22), 6662–6666. https://doi.org/10.1016/j.bmcl.2011.09.061
- Protein Data Bank. Available at: https://www.rcsb.org/ Last accessed: 15.08.2025
- Dileep, K. V., Ihara, K., Mishima-Tsumagari, C., Kukimoto-Niino, M., Yonemochi, M., Hanada, K. et al. (2022). Crystal structure of human acetylcholinesterase in complex with tacrine: Implications for drug discovery. International Journal of Biological Macromolecules, 210, 172–181. https://doi.org/10.1016/j.ijbiomac.2022.05.009
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (2010). Official Journal of the European Union. Available at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF
- Housing and husbandry: Mouse (2024). National Centre for the Replacement, Refinement and Reduction of Animals in Research. Available at: https://nc3rs.org.uk/3rs-resources/housing-and-husbandry-mouse
- Pellegrini, C., D’Antongiovanni, V., Fornai, M., Duranti, E., Baldacci, F., Bernardini, N. et al. (2021). Donepezil improves vascular function in a mouse model of Alzheimer’s disease. Pharmacology Research & Perspectives, 9 (6). https://doi.org/10.1002/prp2.871
- Vogel, H. G. (2007). Drug Discovery and Evaluation: Pharmacological Assays. Berlin Heidelberg New York: Springer-Verlag.
- Semenets, A. P., Suleiman, M. M., Fedosov, A. I., Shtrygol, S. Y., Havrylov, I. O., Mishchenko, M. V. et al. (2022). Synthesis, docking, and biological evaluation of novel 1-benzyl-4-(4-(R)-5-sulfonylidene-4,5-dihydro-1H-1,2,4-triazol-3-yl)pyrrolidin-2-ones as potential nootropic agents. European Journal of Medicinal Chemistry, 244, 114823. https://doi.org/10.1016/j.ejmech.2022.114823
- Podolsky, I., Shtrygol', S. (2019). The behavioral study of the effects of atristamine on the serotonin, dopamine and norepinephrine neurotransmitter systems in mice. Farmacia, 67 (2), 296–304. https://doi.org/10.31925/farmacia.2019.2.14
- Havrylov, I., Shtrygol’, S. (2021). Investigation of the effect of a modified fragment of neuropeptide Y on memory phases and extrapolation escape of animals. Česká a Slovenská Farmacie, 70 (3), 91–99. https://doi.org/10.5817/csf2021-3-91
- Bloch, S., Belzung, C.; Harro, J. (Ed.) (2023). The Light–Dark Box Test in the Mouse. Psychiatric Vulnerability, Mood, and Anxiety Disorders. New York: Humana, 31–41. https://doi.org/10.1007/978-1-0716-2748-8_3
- Takao, K., Miyakawa, T. (2006). Light/dark Transition Test for Mice. Journal of Visualized Experiments, 1. https://doi.org/10.3791/104
- Hock, F. J. (Ed.) (2014). Drug discovery and evaluation: Pharmacological assays. Berlin, Heidelberg: Springer. https://doi.org/10.1007/978-3-642-27728-3
- Moser, V. C. (2010). Functional Assays for Neurotoxicity Testing. Toxicologic Pathology, 39 (1), 36–45. https://doi.org/10.1177/0192623310385255
- Hunskaar, S., Berge, O.-G., Hole, K. (1986). A modified hot-plate test sensitivie to mild analgesics. Behavioural Brain Research, 21 (2), 101–108. https://doi.org/10.1016/0166-4328(86)90088-4
- Grabowska, W., Bijak, M., Szelenberger, R., Gorniak, L., Podogrocki, M., Harmata, P., Cichon, N. (2025). Acetylcholinesterase as a Multifunctional Target in Amyloid-Driven Neurodegeneration: From Dual-Site Inhibitors to Anti-Agregation Strategies. International Journal of Molecular Sciences, 26 (17), 8726. https://doi.org/10.3390/ijms26178726
- Zhang, J., Wang, J., Zhou, G.-S., Tan, Y.-J., Tao, H.-J., Chen, J.-Q. et al. (2019). Studies of the Anti-amnesic Effects and Mechanisms of Single and Combined Use of Donepezil and Ginkgo Ketoester Tablet on Scopolamine-Induced Memory Impairment in Mice. Oxidative Medicine and Cellular Longevity, 2019, 1–16. https://doi.org/10.1155/2019/8636835
- Papp, M., Gruca, P., Lason-Tyburkiewicz, M., Willner, P. (2016). Antidepressant, anxiolytic and procognitive effects of rivastigmine and donepezil in the chronic mild stress model in rats. Psychopharmacology, 233 (7), 1235–1243. https://doi.org/10.1007/s00213-016-4206-0
- Selvy, M., Mattévi, C., Dalbos, C., Aissouni, Y., Chapuy, E., Martin, P.-Y. et al. (2022). Analgesic and preventive effects of donepezil in animal models of chemotherapy-induced peripheral neuropathy: Involvement of spinal muscarinic acetylcholine M2 receptors. Biomedicine & Pharmacotherapy, 149, 112915. https://doi.org/10.1016/j.biopha.2022.112915
- Pelsőczi, P., Lévay, G. (2017). Effect of Scopolamine on Mice Motor Activity, Lick Behavior and Reversal Learning in the IntelliCage. Neurochemical Research, 42 (12), 3597–3602. https://doi.org/10.1007/s11064-017-2408-4
- Montigné, E., Balayssac, D. (2023). Exploring Cholinergic Compounds for Peripheral Neuropathic Pain Management: A Comprehensive Scoping Review of Rodent Model Studies. Pharmaceuticals, 16 (10), 1363. https://doi.org/10.3390/ph16101363
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Rita Saifudinova, Hanna Severina, Sergiy Vlasov, Georgiy Yakovenko, Andrii Khairulin, Dmytro Kyrylov, Mykyta Нutorka, Sergii Shtrygol’, Victoriya Georgiyants

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






