Study of thermogravimetric profile of a mixture of diclofenac sodium and para-aminobenzoic acid as a basis for pharmaceutical development
DOI:
https://doi.org/10.15587/2519-4852.2025.347089Keywords:
derivatography, thermogravimetry, stability, compatibility, thermal decomposition, active pharmaceutical ingredients, diclofenac sodium, para-aminobenzoic acid, thermal analysisAbstract
In modern conditions, in particular, against the background of an increase in the frequency of traumatic injuries to the organs of vision because of hostilities and the influence of professional factors, the development of new ophthalmic dosage forms is of particular relevance. Combined drugs containing several active pharmaceutical ingredients, in particular diclofenac sodium and para-aminobenzoic acid, are considered promising solutions for the treatment of infectious and inflammatory eye lesions. At the same time, the effectiveness and safety of such combinations directly depend on their physicochemical compatibility, which determines the stability of the finished dosage form.
The aim of the study is to investigate the thermal stability and possible physicochemical interactions between diclofenac sodium and para-aminobenzoic acid by derivatographic analysis of individual substances and their mixture in a 1:1 ratio.
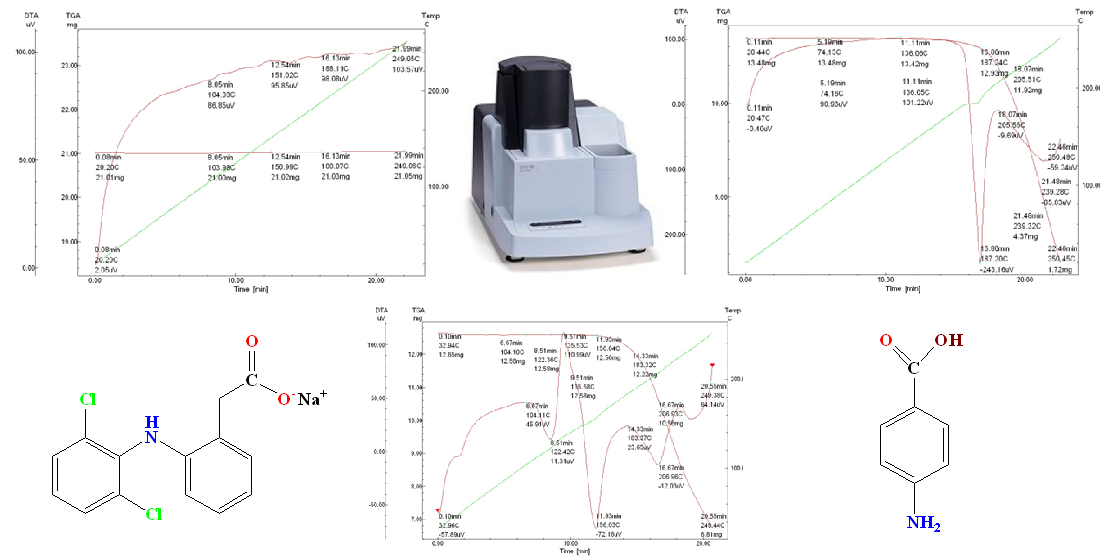
Materials and methods. The objects of the study were the pharmaceutical substances diclofenac sodium, para-aminobenzoic acid and a model mixture of the specified active pharmaceutical ingredients. Thermogravimetric analysis was carried out on a Shimadzu DTG-60 device in thermogravimetric analysis and differential thermal analysis modes in the temperature range of 17–250 °C with a heating rate of 10 °C/min in an air atmosphere.
Results. In the process of thermogravimetric research, it was established that diclofenac sodium is characterized by high thermal stability. Para-aminobenzoic acid showed three stages of thermal destruction, but analysis of the mixture in a 1:1 ratio did not reveal the appearance of new thermal effects or shifts in the degradation profiles.
Conclusions. The results of thermogravimetric analysis indicate the compatibility of diclofenac sodium and para-aminobenzoic acid in terms of thermal characteristics, which confirms the prospects of their simultaneous use
References
- Kucherenko, L. I., Okolzin, D. V. (2025). The relevance of creating a new combined ophthalmic drug with anti-inflammatory and antibacterial effects. Current Issues in Pharmacy and Medicine: Science and Practice, 18 (2), 215–222. https://doi.org/10.14739/2409-2932.2025.2.325216
- Frolova, Y., Kaplaushenko, A., Nagornaya, N. (2020). Design, synthesis, antimicrobial and antifungal activities of new 1,2,4-triazole derivatives containing 1H-tetrazole moiety. Ankara Universitesi Eczacilik Fakultesi Dergisi, 44 (1), 70–88. https://doi.org/10.33483/jfpau.574001
- Tanihara, H., Yamamoto, T., Aihara, M., Koizumi, N., Minami, H., Kojima, S. et al. (2023). Crossover Randomized Study of Pharmacologic Effects of Ripasudil–Brimonidine Fixed-Dose Combination Versus Ripasudil or Brimonidine. Advances in Therapy, 40 (8), 3559–3573. https://doi.org/10.1007/s12325-023-02534-w
- Kucherenko, L., Nimenko, G., Khromylova, O., Borsuk, S. (2022). Validation of quantitative determination methods of active substances in Carbatryl tablets. Research Journal of Pharmacy and Technology, 15 (11), 5148–5153. https://doi.org/10.52711/0974-360x.2022.00866
- Kucherenko, L., Nimenko, H., Khromylova, O., Borsuk, S., Belenichev, I., Hura, E. (2023). Validation of the method of standardization of concomitant impurities in Carbatryl tablets. Research Journal of Pharmacy and Technology, 16 (9), 4415–4422. https://doi.org/10.52711/0974-360x.2023.00721
- Kucherenko, L., Derevianko, N., Borsuk, S., Khromylova, O., Bihdan, O., Skoryna, D. (2025). Development of an Optimal Method for the Quantitative Determination of 1-(ß phenylethyl)-4-amino-1,2,4-triazolium Bromide in a solution for Injection. Research Journal of Pharmacy and Technology, 18 (7), 2998–3002. https://doi.org/10.52711/0974-360x.2025.00429
- Fuwa, M., Shimazaki, A., Odani-Kawabata, N., Kirihara, T., Taniguchi, T., Iwamura, R. et al. (2021). Additive Intraocular Pressure-Lowering Effects of a Novel Selective EP2 Receptor Agonist, Omidenepag Isopropyl, Combined with Existing Antiglaucoma Agents in Conscious Ocular Normotensive Monkeys. Journal of Ocular Pharmacology and Therapeutics, 37 (4), 223–229. https://doi.org/10.1089/jop.2020.0071
- Weekes, L., Ramzan, I. (2021). Prescription of compounded ophthalmic medications – a pharmacy perspective. Clinical and Experimental Optometry, 104 (3), 406–411. https://doi.org/10.1111/cxo.13066
- Hui, A., Jalbert, I. (2021). Ocular therapeutics: from special interest to standard care. Clinical and Experimental Optometry, 104 (3), 265–266. https://doi.org/10.1080/08164622.2021.1877535
- Sipos, P., Szűcs, M., Szabó, A., Erős, I., Szabó-Révész, P. (2008). An assessment of the interactions between diclofenac sodium and ammonio methacrylate copolymer using thermal analysis and Raman spectroscopy. Journal of Pharmaceutical and Biomedical Analysis, 46 (2), 288–294. https://doi.org/10.1016/j.jpba.2007.10.008
- Tudja, P., Khan, M. Z. I., Meštrovic, E., Horvat, M., Golja, P. (2001). Thermal Behaviour of Diclofenac Sodium: Decomposition and Melting Characteristics. Chemical and Pharmaceutical Bulletin, 49 (10), 1245–1250. https://doi.org/10.1248/cpb.49.1245
- Freitas, E. D., Vidart, J. M. M., da Silva, M. G. C., Vieira, M. G. A. (2021). Thermal characterization and stability investigation of sericin and alginate blend loaded with diclofenac sodium or ibuprofen. European Polymer Journal, 142, 110125. https://doi.org/10.1016/j.eurpolymj.2020.110125
- Kenawi, I. M. (2005). Density functional theory assessment of the thermal degradation of diclofenac and its calcium and iron complexes. Journal of Molecular Structure, 754 (1-3), 61–70. https://doi.org/10.1016/j.molstruc.2005.06.021
- Dang, D. H., Riaz, K. M., Karamichos, D. (2022). Treatment of Non-Infectious Corneal Injury: Review of Diagnostic Agents, Therapeutic Medications, and Future Targets. Drugs, 82 (2), 145–167. https://doi.org/10.1007/s40265-021-01660-5
- Bai, R., Liu, L., Chen, Z., Ma, Q. (2022). Cyclosporine (0.05%) Combined with Diclofenac Sodium Eye Drops for the Treatment of Dry Eye Disease. Journal of Ophthalmology, 2022, 1–6. https://doi.org/10.1155/2022/2334077
- Owji, A. P., Dong, J., Kittredge, A., Wang, J., Zhang, Y., Yang, T. (2024). Neurotransmitter-bound bestrophin channel structures reveal small molecule drug targeting sites for disease treatment. Nature Communications, 15 (1). https://doi.org/10.1038/s41467-024-54938-z
- Galbinur, T., Obolensky, A., Berenshtein, E., Vinokur, V., Chowers, I., Chevion, M., Banin, E. (2009). Effect of Para-Aminobenzoic Acid on the Course of Retinal Degeneration in the rd10 Mouse. Journal of Ocular Pharmacology and Therapeutics, 25 (6), 475–482. https://doi.org/10.1089/jop.2009.0020
- Teixeira, J. A., Nunes, W. D. G., Colman, T. A. D., do Nascimento, A. L. C. S., Caires, F. J., Campos, F. X. et al. (2016). Thermal and spectroscopic study to investigate p-aminobenzoic acid, sodium p-aminobenzoate and its compounds with some lighter trivalent lanthanides. Thermochimica Acta, 624, 59–68. https://doi.org/10.1016/j.tca.2015.11.023
- Gupta, K. R., Pounikar, A. R., Umekar, M. J. (2019). Drug Excipient Compatibility Testing Protocols and Charaterization: A Review. Asian Journal of Chemical Sciences, 6 (3), 1–22. https://doi.org/10.9734/ajocs/2019/v6i319000
- Jain, S., Shah, R. P. (2023). Drug-Excipient Compatibility Study Through a Novel Vial-in-Vial Experimental Setup: A Benchmark Study. AAPS PharmSciTech, 24 (5). https://doi.org/10.1208/s12249-023-02573-0
- Ramos, P. (2022). Application of Thermal Analysis to Evaluate Pharmaceutical Preparations Containing Theophylline. Pharmaceuticals, 15 (10), 1268. https://doi.org/10.3390/ph15101268
- Marianni, B., Silva, C. C. V., Polonini, H. (2025). Compatibility of active pharmaceutical ingredients combinations compounded in Cleoderm™, a cream base for personalized dermatological treatments. International Journal of Pharmaceutical Compounding, 29(2), 150–162.
- Broncel, M., Juszczak, A., Szczolko, W., Silvestri, D., Białek-Dratwa, A., Wacławek, S. et al. (2024). Thermal Compatibility of New ACEI Derivatives with Popular Excipients Used to Produce Solid Pharmaceutical Formulations. Pharmaceuticals, 17 (10), 1323. https://doi.org/10.3390/ph17101323
- Saadatkhah, N., Carillo Garcia, A., Ackermann, S., Leclerc, P., Latifi, M., Samih, S. et al. (2019). Experimental methods in chemical engineering: Thermogravimetric analysis—TGA. The Canadian Journal of Chemical Engineering, 98 (1), 34–43. https://doi.org/10.1002/cjce.23673
- Costa, S. P. M., da Silva, K. E. R., de Medeiros, G. C. R., Rolim, L. A., de Oliveira, J. F., de Lima, M. do C. A. et al. (2013). Thermal behavior and compatibility analysis of the new chemical entity LPSF/FZ4. Thermochimica Acta, 562, 29–34. https://doi.org/10.1016/j.tca.2013.03.003
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Liudmila Kucherenko, Bohdan Burlaka, Serhii Borsuk, Oleksii Bihdan, Volodymyr Parchenko, Dmytro Skoryna, Ivan Pavliuk

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






