Development and validation of an HPLC method for the determination of glucosamine hydrochloride in a medicinal product in the form of a cream
DOI:
https://doi.org/10.15587/2519-4852.2025.347870Keywords:
glucosamine hydrochloride, cream, derivatization, HPLC, validation, quality controlAbstract
The development of medicinal products requires the use of selective and reproducible analytical methods that ensure reliable determination of active ingredients. The analysis of glucosamine hydrochloride as a medicinal product in the form of a cream has specific challenges due to its high polarity and lack of a chromophore; therefore, its determination within the dosage form requires a specially adapted analytical approach.
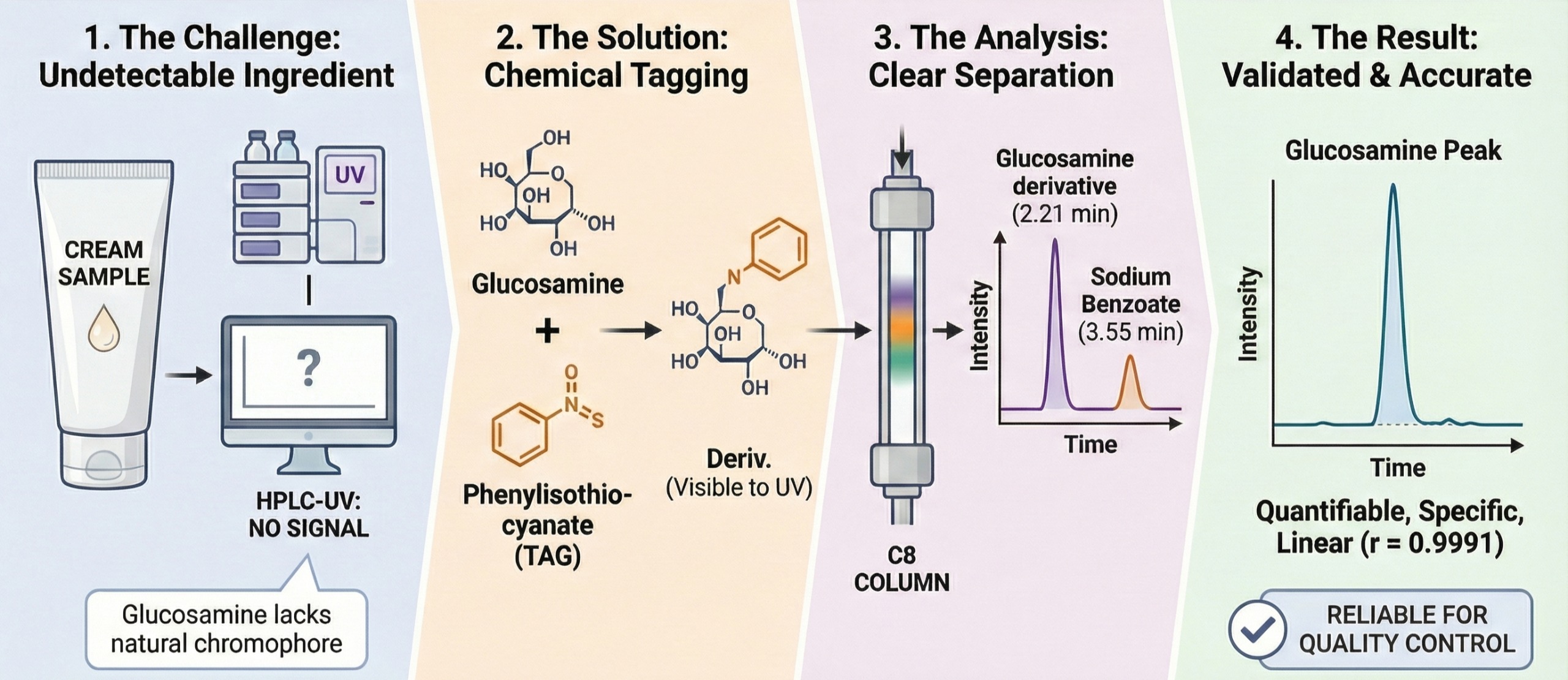
The aim. To develop and validate a liquid chromatographic method for the determination of glucosamine hydrochloride in a medicinal product in the form of a cream in accordance with the requirements of ICH Q2(R2), the State Pharmacopoeia of Ukraine (SPhU), and the European Pharmacopoeia (EP).
Materials and methods. The object of the study was an experimental batch of a cream containing glucosamine hydrochloride (1%). The analysis was performed using a KNAUER Smartline chromatograph equipped with a UV detector and a Zorbax SB-C8 column (150 × 4.6 mm, 5 μm). Phenyl isothiocyanate was used for derivatization, resulting in the formation of a UV-active glucosamine derivative. Validation was carried out with respect to specificity, linearity, accuracy, precision, system suitability, and robustness.
Results. Optimal chromatographic conditions were established (mobile phase: acetonitrile–water, 40:60, acidified with phosphoric acid to pH 3.0; detection wavelength 230 nm; flow rate 1.0 mL/min; column thermostat temperature 25°C), ensuring complete separation of the glucosamine derivative (retention time 2.21 min) from sodium benzoate (retention time 3.55 min) and other excipients in the cream formulation. The minimum required concentration of the derivatization reagent was determined to be 0.008–0.01 g/mL. Evaluation of validation parameters confirmed the specificity of the method, its linearity within the 80–120% range (r = 0.9991), precision (ΔZ = 0.61%), accuracy (δ = 0.31%), repeatability (≤ 2%), and robustness (analytical solutions stable for 1 hour). Metrological assessment (n = 6) demonstrated that the systematic error was statistically insignificant, while the relative uncertainty of a single determination was 7.88% at a confidence level of P = 0.95. The detection limit of glucosamine hydrochloride is 0.23 μg/ml.
Conclusions. An HPLC method for the determination of glucosamine hydrochloride in a cream formulation after derivatization with phenyl isothiocyanate has been proposed and experimentally substantiated. The method complies with the requirements of international guidelines and can be applied in quality control for the identification and quantitative determination of the active pharmaceutical ingredient in the investigated medicinal product
Supporting Agency
- “Development of topical pharmaceutical products for improving the functional condition of the stump during prosthetics” financed by the Ministry of Health of Ukraine (Order of the Ministry of Health of Ukraine dated February 6, 2025, No. 208).
References
- Shareef, U., Altaf, A., Ahmed, M., Akhtar, N., Almuhayawi, M. S., Al Jaouni, S. K. et al. (2024). A comprehensive review of discovery and development of drugs discovered from 2020–2022. Saudi Pharmaceutical Journal, 32 (1), 101913. https://doi.org/10.1016/j.jsps.2023.101913
- Rudakova, O., Gubar, S., Smielova, N., Lytkin, D., Briukhanova, T., Bezchasnyuk, E. et al. (2021). Study of compatibility of components of a new combined drug for treatment of alcoholic intoxication and its hepatoprotective effect on a model of alcoholic liver injury. ScienceRise: Pharmaceutical Science, 6 (34), 91–100. https://doi.org/10.15587/2519-4852.2021.249880
- Rudakova, O., Gubar, S., Smielova, N., Yaremenko, M., Bevz, N., Georgiyants, V. (2022). Development of a unified approach to the method of identification, quantitative determination of active substances and accompanying impurities in a combined drug by HPLC method. ScienceRise: Pharmaceutical Science, 2(36), 81–89. https://doi.org/10.15587/2519-4852.2022.255851
- Kumar, D., Kumar, A., Kumar, V., Raj, A., Rai, R. R. M., Baliyan, V., Kumar, N. (2023). A Comprehensive Review on Analytical Method Development using RP-HPLC and Recent Advances in Pharmaceutical Applications. Journal for Research in Applied Sciences and Biotechnology, 2 (2), 53–60. https://doi.org/10.55544/jrasb.2.2.9
- Ankit, J., Vijayraj, S. (2024). Advancements in Analytical Development and Validation: A Comprehensive Review in Pharmaceutical Sciences. Open Access Journal of Pharmaceutical Research, 8 (3). https://doi.org/10.23880/oajpr-16000317
- Chaudhary, T., Kumar, A., Raj, N. D., Sarma, G., Dawange, S., Singh, D. (2024). An Exhaustive Review on Recent Trends in Analytical Methods: Development Strategies and Recent Applications. Current Indian Science, 2. https://doi.org/10.2174/012210299x24646823111505171
- Savchenko, L., Uminska, K., Bevz, N., Georgiyants, V. (2018). Selection and verification of the method for phynelefrine hydrochloride assay in Simanovsky ointment. ScienceRise: Pharmaceutical Science, 1 (11), 26–31. https://doi.org/10.15587/2519-4852.2018.122094
- Ruban, O., Slipchenko, H., Osolodchenko, T., Kovalevska, I., Khokhlenkova, N. (2025). Research on the antimicrobial preservative selection for the cream containing glucosamine hydrochloride and miramistin. Annals of Mechnikov Institute, 3, 85–91. https://doi.org/10.5281/zenodo.17105302
- ICH Q14 – Analytical Procedure Development (2023). International Council for Harmonisation (ICH). Available at: https://www.ema.europa.eu/en/ich-q14-analytical-procedure-development-scientific-guideline
- Derzhavna Farmakopeia Ukrainy. Dopovnennia 8 (2025). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 452.
- European Pharmacopoeia (EP). (n.d.). European Directorate for the Quality of Medicines.
- The British Pharmacopoeia. (2023). Available at: https://www.pharmacopoeia.com/
- Derzhavna Farmakopeia Ukrainy. Dopovnennia 5. (2021). Kharkiv: DP «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 424.
- Gaonkar, P., Khanvilkar, V., Shettigar, R., Gadgoli, C. (2006). Spectrophotometric method for determination of glucosamine in tablets. Indian Journal of Pharmaceutical Sciences, 68, 83–84.
- Derzhavna Farmakopeia Ukrainy. Dopovnennia 7. Vol. 2 (2024). Kharkiv: DP «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 424.
- El-Saharty, Y. S., Bary, A. A. (2002). High-performance liquid chromatographic determination of neutraceuticals, glucosamine sulphate and chitosan, in raw materials and dosage forms. Analytica Chimica Acta, 462 (1), 125–131. https://doi.org/10.1016/s0003-2670(02)00279-9
- Alcázar Magaña, A., Wrobel, K., Corrales Escobosa, A. R., Wrobel, K. (2015). Determinación rápida de glucosamina en formulaciones farmacéuticas mediante cromatografía líquida de alta resolución sin derivatización pre-columna. Acta Universitaria, 24, 16–22. https://doi.org/10.15174/au.2014.717
- Pazourek, J. (2018). Determination of glucosamine and monitoring of its mutarotation by hydrophilic interaction liquid chromatography with evaporative light scattering detector. Biomedical Chromatography, 32 (12). https://doi.org/10.1002/bmc.4368
- Asthana, C., Peterson, G. M., Shastri, M., & Patel, R. P. (2019). Development and validation of a novel high performance liquid chromatography-coupled with Corona charged aerosol detector method for quantification of glucosamine in dietary supplements. PLoS One, 14(5), e0216039. https://doi.org/10.1371/journal.pone.0216039
- Šulc, M. (2024). Chitin quantitation (as glucosamine) in food raw materials by HPLC C18-DAD with off-line derivatization. MethodsX, 12, 102729. https://doi.org/10.1016/j.mex.2024.102729
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nataliia Bevz, Yaroslav Studenyak, Olena Ivaniuk, Olena Ruban

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






